

A research team led by Distinguished Professor Hao Li at WPI-AIMR, Tohoku University has reported new findings on copper/cobalt-based catalysts that improve the efficiency of electrochemical nitrate reduction. The study addresses a key rate-limiting step in the conversion of nitrate (NO3−) to ammonia (NH3), offering a refined approach to green ammonia production and nitrate wastewater treatment.
Electrochemical nitrate reduction (NO3−RR) is emerging as a viable strategy for producing ammonia under ambient conditions while also mitigating nitrate pollution. However, the multi-step process is often hindered by the slow conversion of nitrate to nitrite (NO2−), which can significantly reduce overall efficiency.
In this work, the researchers synthesized spherical and nanoflower-like CuO/CuCo2O4 catalysts using an emulsion hydrothermal method. The design promotes small-particle stacking and leverages the structural benefits of both CuO and Co3O4. The catalysts were shown to facilitate the sequential reaction steps, effectively connecting NO3−→ NO2− and NO2−→ NH3 in a unified system.
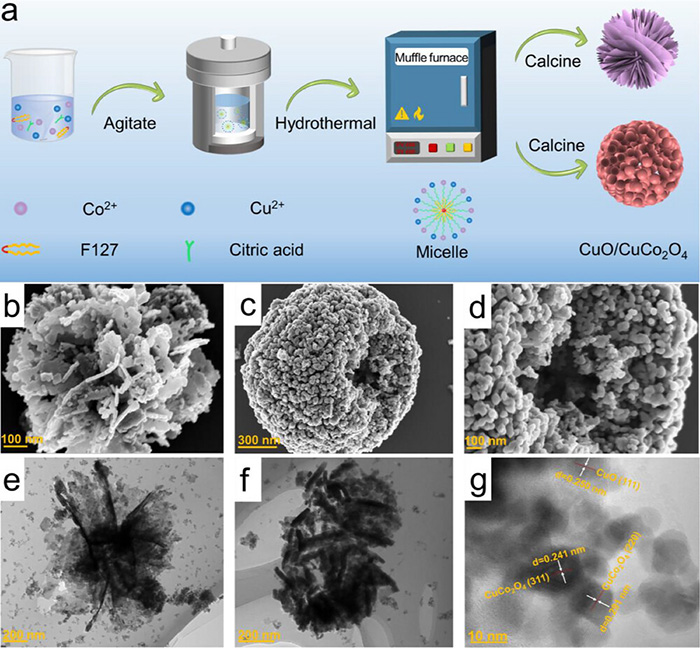
Synthesis route of CuCo2O4; b–d) FE-SEM, e,f) TEM, g) HR-TEM of 4-CuCo2O4. ©Yuan Wang et al.
One of the study’s central findings is the formation of monomeric copper during electrolysis. This newly formed Cu interacts with CuCo2O4 to promote the rate-limiting NO3−→ NO2− step. As a result, the same level of ammonia production was observed in both nitrate and nitrite reduction reactions.
Under neutral conditions at −0.70 V (vs. RHE), the Cu/CuCo2O4 catalyst achieved a peak ammonia yield of 24.58 mg h−1 mgcat−1 in nitrate reduction, alongside a Faraday efficiency of 100%. When NO2− was used as the substrate, the system achieved a nearly identical yield of 24.34 mg h−1 mgcat−1.
“The ability of Cu and CuCo2O4 to work in tandem helps us better understand how to design more effective catalysts,” said Professor Hao Li. “Our findings provide not only experimental results but also mechanistic insights that may guide future catalyst development.”
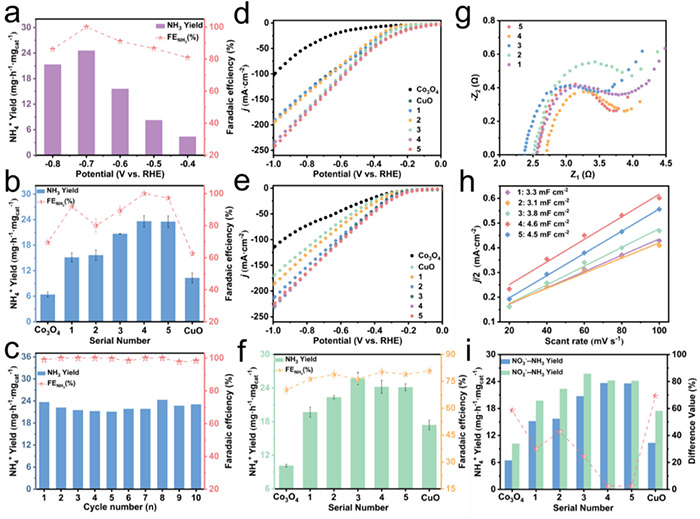
a) Ammonia yield and FE at different potentials of 4-CuCo2O4; b) Ammonia yield and FE of different samples at −0.70 V (vs RHE), 0.1 M NO3− + 0.5 M Na2SO4; c) CA stabilization cycle; d,e) LSV of different samples at 0.1 M NO3− / NO2− + 0.5 M Na2SO4, respectively; f) Ammonia yield and FE of different samples at −0.70 V (vs RHE), 0.1 M NO2− + 0.5 M Na2SO4; g) EIS of different samples; h) ECSA of different samples; i) Difference in ammonia yield of different samples at NO3−RR and NO2−RR. ©Yuan Wang et al.
The team has made the experimental and computational data available through the Digital Catalysis Platform (DigCat) a database developed by the Hao Li laboratory. These results contribute to ongoing efforts to optimize catalysts for sustainable ammonia production.
a database developed by the Hao Li laboratory. These results contribute to ongoing efforts to optimize catalysts for sustainable ammonia production.
Future work will focus on extending the catalyst’s performance to industrial settings, including long-duration tests and reactor-scale implementation, while deepening the mechanistic understanding through operando spectroscopy and modeling.
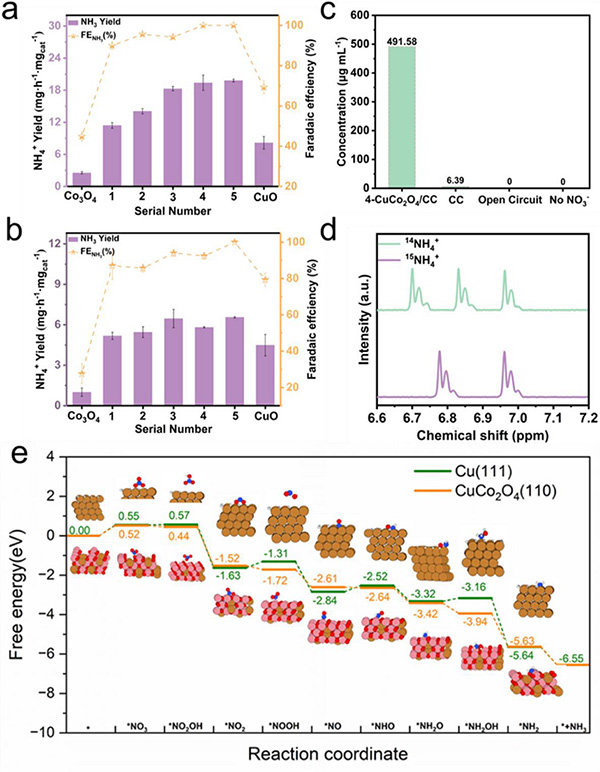
a) Ammonia yield and FE of different samples at −0.70 V (vs RHE), 0. 5 M Na2SO4 + 0.05 M NO3−; b) Ammonia yield and FE of different samples at −0.70 V (vs RHE), 0.5 M Na2SO4 + 0.01 M NO3−; c) NH3 concentration of 4-CuCo2O4/CC in the presence or absence of NO3−, CC, and open potential conditions at −0.70 V (vs RHE); d) 1H-NMR of 15NH4+ and 14NH4+; e) Calculated free energy diagram of NO3−RR on Cu (1 1 1) and CuCo2O4(1 1 0). ©Yuan Wang et al.
| タイトル: | Advancing Electrochemical Nitrate Reduction: Overcoming Rate-Limiting Bottlenecks with Copper/Cobalt Catalysts |
|---|---|
| 著者: | Jin Li, Yuan Wang, Xiujing Xing, Yang Wang, Wei Xiong, and Hao Li |
| 掲載誌: | Advanced Functional Materials |
| DOI: | 10.1002/adfm.202513717 |
東北大学材料科学高等研究所(WPI-AIMR)
教授 Hao Li(研究者プロフィール)
| E-mail: | li.hao.b8@tohoku.ac.jp |
|---|---|
| Webstie: | Hao Li Laboratory |
東北大学材料科学高等研究所(WPI-AIMR) 広報戦略室
| Tel: | 022-217-6146 |
|---|---|
| E-mail: | aimr-outreach@grp.tohoku.ac.jp |