

A research team has unveiled a breakthrough in improving the performance of zinc-air batteries (ZABs), which are an important energy storage technology. This breakthrough involves a new catalyst that significantly boosts the efficiency of the oxygen reduction reaction (ORR), a crucial process in ZABs. The development could lead to more efficient, long-lasting batteries for practical applications.
The oxygen reduction reaction is a critical step in many energy conversion devices, including ZABs. However, the reaction often suffers from slow kinetics, which limits the performance of the batteries. To solve this, platinum-based catalysts are typically used, but they are expensive, scarce, and can be poisoned by impurities. Researchers have been searching for alternatives that are both cost-effective and highly efficient. This study focuses on a new class of catalysts called dual-atom catalysts (DACs), which consist of two metal atoms closely paired together to enhance catalytic activity.
The team, led by Di Zhang, assistant professor at Tohoku University's Advanced Institute of Materials Research (WPI-AIMR), used a combination of computational modeling and experimental techniques to design and create a dual-atom catalyst made of iron (Fe) and cobalt (Co), which are combined with nitrogen (N) and carbon (C) in a porous structure. This catalyst, named Fe1Co1-N-C, was identified as the optimal catalyst for the oxygen reduction reaction in alkaline conditions. The unique combination of materials allows the catalyst to function efficiently, making it a promising candidate for use in ZABs.
The researchers designed the Fe1Co1-N-C catalyst by first using a model to predict how pH (acidity) affects the reaction. This guided them in the creation of a catalyst with the right properties for maximum efficiency. They then synthesized the catalyst using a method that involved hard templates and a CO2 activation process to create a structure that has small pores. These pores are essential for allowing reactants to move through the material, which improves the overall catalytic performance.
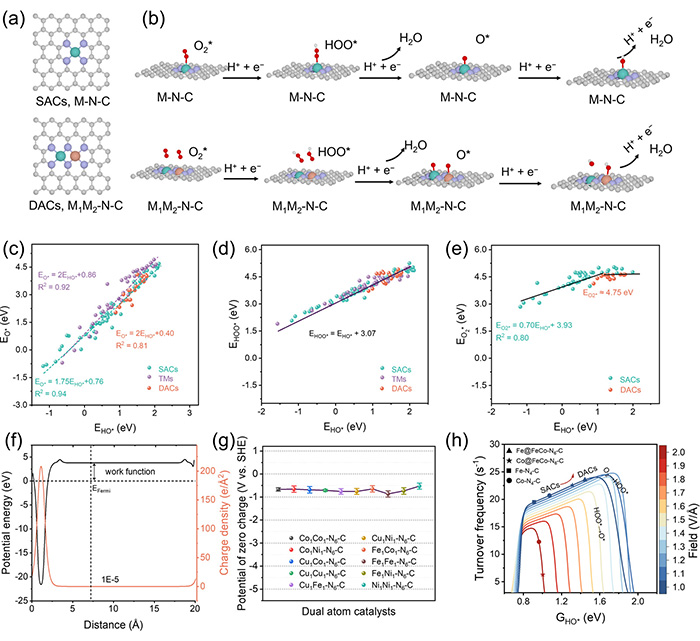
pH-field coupled microkinetic modeling of DACs in ORR. (a) The typical atomic configuration of SACs and DACs. The distance between the two metal atoms is approximately 2.3 Å in DACs. M = Mn, Fe, Co, Ni, or Cu. (b) ORR mechanisms on SACs and DACs. Both central atoms in DACs can concurrently undergo the ORR. The bond cutoff between metal atoms and oxygen was set to 2.2 Å. (c) EHO* vs. EO* scaling relation on the above M-N-C catalysts. The slope of DACs is greater than that of SACs, mirroring the behavior observed with surfaces of TMs. (d) Universal scaling relation of EHO* vs. EHOO* on SACs, DACs, and TMs. (e) EHO* vs. EO2* scaling relation on SACs and DACs. (f) The work function of Fe1Co1-N6-C determined via implicit solvation model. (g) PZCs for various DACs with distinct metal centers. (h) pH-field activity volcano for DACs, highlighting enhanced positioning of Fe/Co atop site towards peak activity in alkaline solutions. The electric field from 1.0 to 2.0 V/Å corresponds to a pH from 1.3 to 13.4. (The above data are stored in https://www.digcat.org ). ©Di Zhang et al.
). ©Di Zhang et al.
The results of the study were impressive. The Fe1Co1-N-C catalyst showed a significantly higher oxygen reduction activity than the commonly used platinum catalyst (Pt/C). In practical terms, the Fe1Co1-N-C-based zinc-air batteries demonstrated a high open-circuit voltage of 1.51 volts, meaning they can generate a substantial amount of energy. Additionally, the batteries displayed an energy density of 1079 watt-hours per kilogram of zinc (Wh kgZn-1), which is an excellent measure of energy storage capability.
In addition to the high voltage and energy density, the Fe1Co1-N-C-based batteries also demonstrated an excellent rate capability, meaning they can perform well even when subjected to high current densities—ranging from 2 to 600 milliamps per square centimeter (mA cm-2). More remarkably, the batteries showed an ultra-long lifespan, lasting over 3600 hours and completing 7200 cycles under a moderate current, which is far superior to most other batteries.
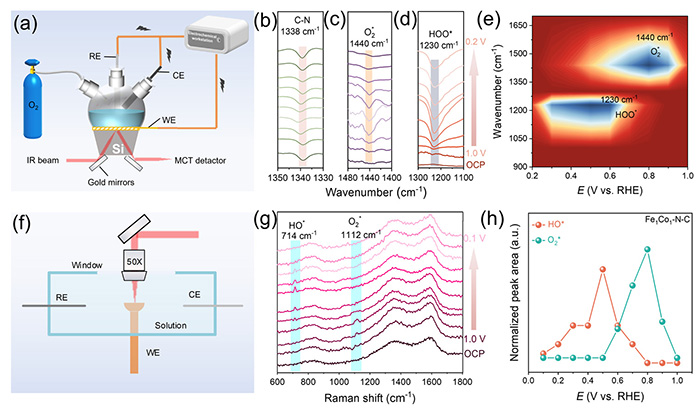
In-situ ATR-SEIRAS and in-situ Raman studies for ORR mechanism. (a, f) Scheme of in-situ ATR-SEIRAS and in-situ Raman spectroscopy equipment. The working electrode, reference electrode, and counter electrode are abbreviated as WE, RE, and CE, respectively. (b-d, g) In-situ ATR-SEIRAS, and Raman spectra were recorded at different potentials for the Fe1Co1-N-C catalyst during ORR process. Peak area variations were taken from in-situ (e) ATR-SEIRAS and (h) Raman spectra for Fe1Co1-N-C. Potential dependence of the normalized peak area of (e) In-situ ATR-SEIRAS for O2* (1400 cm-1) and HOO* (1230 cm-1), and (h) in-situ Raman for O2* (1112 cm-1) and HO* (714 cm-1). ©Di Zhang et al.
Zhang explains, “This work provides an efficient and rational strategy for designing and synthesizing catalysts that can be used in real-world applications. By combining theoretical models with practical synthesis methods, we were able to develop a catalyst that can significantly improve the performance of zinc-air batteries.”
Looking ahead, Zhang and his team plan to continue their research by developing even more advanced methods to create dual-atom catalysts with precise atomic pairings. They also intend to enhance techniques for identifying the specific active sites in the catalysts. These efforts aim to further optimize the performance of energy conversion technologies and make them even more efficient and cost-effective for widespread use.
The research was supported by the Tohoku University Support Program, and key findings from this study are available on the Digital Catalysis Platform (DigCat) , a resource developed by the Hao Li Lab to assist in the discovery and development of new catalysts.
, a resource developed by the Hao Li Lab to assist in the discovery and development of new catalysts.
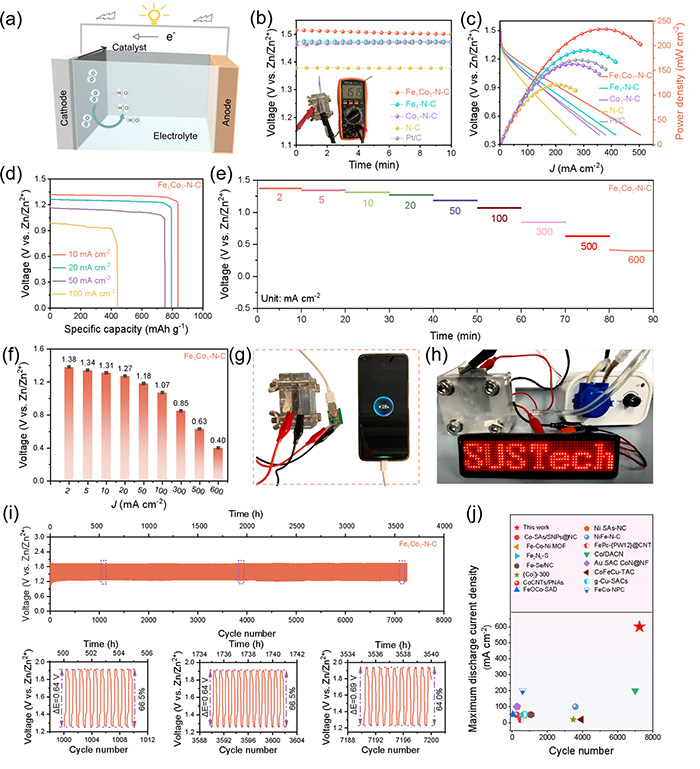
ZAB performance. (a) Schematic illustration of a ZAB. (b) The OCV plots of the ZABs based on Fe1Co1-N-C and reference catalysts (Fe1-N-C, Co1-N-C, N-C, and Pt/C). (c) Discharge polarization plots and corresponding power density plots of the ZABs. (d) Specific capacities of the ZAB based on Fe1Co1-N-C at various current densities (10, 20, 50, and 100 mA cm-2). (e, f) Discharge curves at current densities from 2 to 5, 10, 20, 50, 100, 300, 500, 600 mA cm-2 for Fe1Co1-N-C based ZAB, and voltage values corresponding to each current density. (g, h) Optical images of a phone and an LED light powered by the Fe1Co1-N-C based ZAB. (i) Cyclic stability at 5 mA cm-2 current density for Fe1Co1-N-C based ZAB. (j) Recently reported ZAB performance about their corresponding maximum discharge current density and cycle time. ©Di Zhang et al.
| タイトル: | A pH-dependent microkinetic modeling guided synthesis of porous dual-atom catalysts for efficient oxygen reduction in Zn-air batteries |
|---|---|
| 著者: | Tingting Li, Di Zhang, Yun Zhang, Danli Yang, Runxin Li, Fuyun Yu, Kengqiang Zhong, Xiaozhi Su, Tianwei Song, Long Jiao, Hai-Long Jiang, Guo-Ping Sheng, Jie Xu, Hao Li, Zhen-Yu Wu |
| 掲載誌: | Energy & Environmental Science |
| DOI: | 10.1039/D5EE00215J |
東北大学材料科学高等研究所(WPI-AIMR)
助教 Di Zhang(研究者プロフィール)
| E-mail: | di.zhang.a8@tohoku.ac.jp |
|---|
東北大学材料科学高等研究所(WPI-AIMR)
教授 Hao Li(研究者プロフィール)
| E-mail: | li.hao.b8@tohoku.ac.jp |
|---|---|
| Webstie: | Hao Li Laboratory |
東北大学材料科学高等研究所(WPI-AIMR) 広報戦略室
| Tel: | 022-217-6146 |
|---|---|
| E-mail: | aimr-outreach@grp.tohoku.ac.jp |