

The hydrogen evolution reaction (HER) is a remarkable process that can create clean hydrogen fuel – a potential part of a solution to our climate change crisis. The problem lies in scaling up this reaction from a lab experiment to largescale commercial production, while keeping costs down.
The findings were published in Advanced Energy Materials on April 3, 2025.
In their search for superior HER performance, researchers at Tohoku University demonstrated that a surface reconstruction pathway can produce durable non-noble metal-based cathodes that speed up the HER reaction. They can maintain their performance for more than 300 hours and are calculated to cost very close to the US Department of Energy’s 2026 H2 production target ($2.00 per kgH2−1). This could pave the way for the rational design of brand new, highly-efficient non-noble metal-based cathodes for commercial PEM application – finally bridging the gap from laboratory to factory.
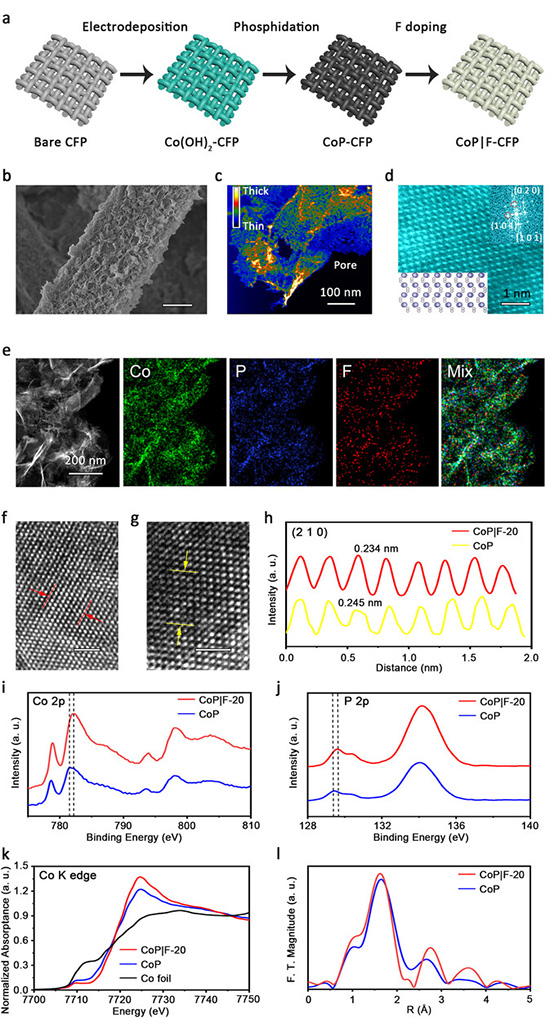
Characterizations of CoP|F-20 and CoP. a) Schematic synthetic illustration of CoP|F on CFP. b) SEM image of CoP|F-20 nanosheets on a single carbon fiber. Scale bar, 2 µm. c) A false-color TEM image of a typical CoP|F-20 nanosheet, showing its relative thickness. Scale bar, 100 nm. d) Atomic-resolution STEM images of CoP|F-20. Scale bar, 1 nm. Inset up right shows the corresponding FFT pattern, and down left shows crystal structure along [101̄] zone axis. e STEM-EDX elemental mapping of CoP|F-20, showing the homogeneous distribution of Co (green), P (blue), and F (red). Scale bar, 200 nm. HAADF-STEM images of CoP|F-20 f and CoP g, and corresponding integrated pixel intensities h of spacings along (201) facet. Scale bar, 1 nm. i) Co 2p and j) P 2p XPS spectra of CoP|F-20 and CoP catalysts. k XANES spectra at Co K-edge of CoP|F-20, CoP, and Co foil. l) R-space curve-fitting of EXAFS spectra of CoP|F-20 and CoP. ©Heng Liu et al.
The angle this study approached for trying to improve the HER – which tends to be inefficient and slow by nature – was transition metal phosphides (TMPs). This promising catalyst (which improves the HER’s efficiency) is a durable and cost-effective non-noble metal. However, typically noble metals are used, so there is a knowledge gap about non-noble metals that needs to be filled.
The research team prepared F modified CoP and examined aspects such as its surface reconstruction and true active sites using operando X-ray absorption spectroscopy (XAS) and Raman measurements. Essentially, adding the F in the CoP1-x lattice allows for P-vacancy sites to form on the surface, which leads to more active sites that are able to speed up the HER.
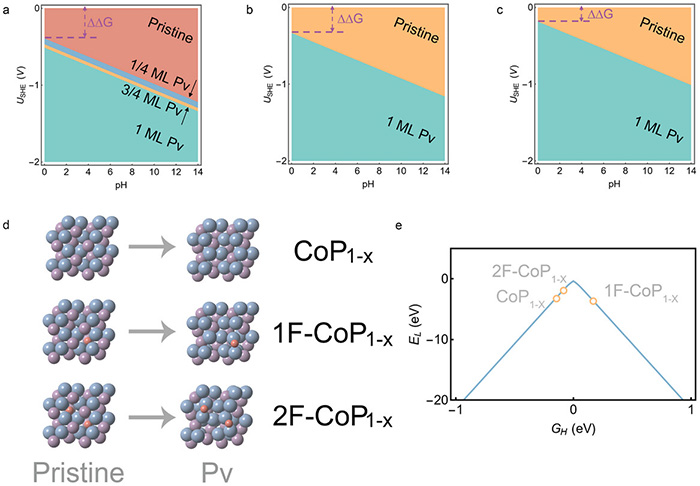
Theoretical calculations on the electrochemistry-induced P-vacancy formation (Pv) and HER activity. a–c) Calculated surface Pourbaix diagrams for a) CoP(010), b) CoP|F(010) with 1F doped at the subsurface, and c) CoP|F(010) with 2F doped at the subsurface. The term ΔΔG refers to the difference in Gibbs free energy between the pristine system and the system after the formation of a phosphorus vacancy. d) The identified surfaces with 1 monolayer Pv formation. Blue, purple, and red spheres represent P, Co, and F, respectively. e) HER volcano activity model showing the theoretical activities of the CoP|F(010) surfaces with Pv. ©Heng Liu et al.
“This reconstructed Co is highly active, works in acidic conditions, and can maintain approximately 76 W for over 300 hours,” says Heng Liu (Advanced Institute for Materials Research (WPI-AIMR)). “We’re getting close an affordable method to produce fuel. The calculated the cost of using this method is $2.17 per kgH2−1 – just 17 cents over the current production target set for 2026.”
The researchers found that when this F modified CoP cathode underwent surface reconstruction, its activity was improved. The experiment doesn’t just test the setup in a lab-scale experimental setup with three electrodes, but also extends the findings to commercial-scale PEM electrolyzers. These results are significant advancements in HER catalyst research that could be the basis for the rational design of other non-noble metal-based cathodes.
“We’re always thinking about the end goal, which is for research to make its way into everyday life. This advancement brings us one step closer to designing more realistic options for commercial PEM application,” says Liu.

PEM test of CoP|F-20 catalyst. a) Schematic illustration of a PEM cell. b) I–V curves of PEM electrolyzers using commercial IrO2 as an anodic catalyst and CoP|F-20 as a cathodic catalyst. No cell voltages were iR compensated. c) Time-dependent power and total H2 generation of PEM electrolyzers using commercial IrO2 as an anodic catalyst and CoP|F-20 as a cathodic catalyst at 1 A cm−2. ©Heng Liu et al.
| タイトル: | Surface Reconstruction Activates Non-Noble Metal Cathode for Proton Exchange Membrane Water Electrolyzer |
|---|---|
| 著者: | Rui Wu, Heng Liu, Jie Xu, Ming-Rong Qu, You-Yi Qin, Xu-Sheng Zheng, Jun-Fa Zhu, Hao Li, Xiao-Zhi Su, Shu-Hong Yu |
| 掲載誌: | Advanced Energy Materials |
| DOI: | 10.1002/aenm.202405846 |
東北大学材料科学高等研究所(WPI-AIMR)
特任助教 Heng Liu(研究者プロフィール)
| E-mail: | heng.liu.e1@tohoku.ac.jp |
|---|
東北大学材料科学高等研究所(WPI-AIMR)
教授 Hao Li(研究者プロフィール)
| E-mail: | li.hao.b8@tohoku.ac.jp |
|---|---|
| Webstie: | Hao Li Laboratory |
東北大学材料科学高等研究所(WPI-AIMR) 広報戦略室
| Tel: | 022-217-6146 |
|---|---|
| E-mail: | aimr-outreach@grp.tohoku.ac.jp |