

02/28/2022
© 2022 Hiroshi Yabu & Patrick Han
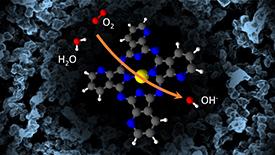
An AIMR-led team has combined synthesis, catalytic-activity and ionization-potential measurements with density functional theory (DFT) simulations to investigate features indicative of high oxygen-reduction reaction (ORR) activities in Katjenblack (KB)-supported metal-azaphthalocyanine (AzPc) catalysts1. The study shows that how the molecule–support hybridization changes the catalyst‘s highest occupied molecular orbital (HOMO) may be a determinant factor in future ORR catalyst designs.
In the race to replace the platinum ORR fuel-cell catalysts, a recent carbon-materials research direction has shifted away from high-temperature pyrolysis treatments—towards fine-structure control.
“Many promising model catalysts have been isolated by hybridizing ORR-active molecules (e.g., metal-AzPc) with carbon conductive agents (e.g., KB),” says Hiroshi Yabu, first author of the study. “What the field needs now are new design principles that can correlate the effects of molecular structure and hybridization with ORR activity.”
Here, the team focuses on the KB-supported metal-AzPc catalyst systems, combining experiments and theory to test the effects of N heteroatom isomers, complex metals such as Fe, Ni, and Cu, and carbon support on ORR activities.
To this end, the team not only synthesizes a series of AzPc molecules with different peripheral structures and complex metals, fabricates the corresponding KB-supported catalysts, and measures their bandgaps and ORR activities, but also performs DFT simulations of ORR processes and reaction pathways.
The combined results indicate that while different complex metals determine the ORR mechanism pathways, how the metal-AzPc–KB interactions change the HOMO of each catalyst determines the ORR activity.
“Comparing Fe, Ni, and Cu as the complex metal, we found that only the Fe-AzPc complexes both favor the 4ē-pathway ORR and enable interactions with the KB support to elevate the catalyst HOMO above the O2 generation potential, promoting ORR,” says Yabu. “These surprising effects should be considered in future ORR catalyst designs.”
Future directions include implementing the electrocatalysts from this study in fuel cells and metal-air batteries, and expanding this study’s approach to catalyst design to other reactions such as oxygen evolution reaction, hydrogen evolution reaction, and CO2 reduction.
(Author: Patrick Han)
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.