

05/25/2020
© 2020 Mingwei Chen
Solid and liquid catalysts in a lithium–oxygen rechargeable battery have a synergistic effect that simultaneously boosts the cathode kinetics and energy efficiency of the battery, researchers at the AIMR have found1. This points the way to overcome two of the main hurdles to the commercialization of these batteries.
“The lithium–oxygen battery is one of the most promising candidates for the next generation of electrochemical energy-storage devices,” says Mingwei Chen of the AIMR at Tohoku University. “But its practical implementation has been hindered by sluggish kinetics at the cathode and low energy efficiencies.”
Lithium–oxygen batteries consist of three main components: a lithium metal anode, a non-aqueous liquid electrolyte and a porous cathode. During discharging, the battery releases energy through the oxidization of lithium to lithium peroxide (Li2O2), and it stores energy during charging by the reverse reaction in which lithium peroxide is decomposed into lithium and oxygen. Catalysts are needed to facilitate both these reactions.
Solid and liquid catalysts have been developed for lithium–oxygen batteries, but they both have their downsides. “Solid catalysts suffer from rigid and unstable catalyst–lithium peroxide interfacial contacts, whereas liquid catalysts have lower catalytic efficiencies,” explains Chen.
Previous studies had shown the promise of using both solid and liquid catalysts for improving the energy efficiency of lithium–oxygen batteries, but until now the underlying mechanisms and the optimal combinations have not been explored.
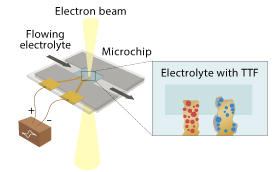
The team led by Chen used a state-of-the-art liquid-cell transmission electron microscope to investigate the effect of using both kinds of catalysts.
Chen and his team chose ruthenium oxide (RuO2) as the solid catalyst and tetrathiafulvalene (C6H4S4) as the liquid catalyst. They then imaged the dynamic nanoscale reactions that took place at the interfaces between the electrode and the electrolyte, and between the electrode and the lithium peroxide (see image).
The researchers discovered a synergistic effect between the two catalysts — in addition to catalyzing lithium–oxygen reactions, the solid catalyst enhanced the catalytic efficiency of the liquid catalyst during discharging. The liquid catalyst activated the lithium peroxide–passivated ruthenium oxide and accelerated the oxidation of lithium peroxide during charging. This synergistic effect boosted the energy efficiency of the rechargeable lithium–oxygen battery.
Chen and his team plan to build upon this work. “Using the electrochemistry insights gained in this study, we intend to work toward developing better combinations of solid and liquid catalysts for high-performance lithium–oxygen batteries,” says Chen.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.