

01/27/2020
© 2020 Mingwei Chen
Researchers at the AIMR have observed how an ultrathin layer on the negative electrode (anode) of lithium-ion batteries forms, grows and fails during battery operation1. The important insights they have gleaned will be useful for developing better and safer lithium-ion batteries.
Lithium-ion batteries power everything from smart phones to electric vehicles. First commercialized about 30 years ago, they are a mature technology that has been intensively studied and developed. However, one of the most important but least understood aspects of lithium-ion batteries is an ultrathin layer — the solid electrolyte interphase (SEI) — that grows on the anode and protects it.
The SEI forms during the first few charge−discharge cycles and protects the anode from further decomposition. Although only a few tens of nanometers thick, the layer has a major effect on battery operation, notes Mingwei Chen at the AIMR of Tohoku University. “The SEI is one of the key factors that determine the safety, power capability and cycle life of lithium-ion batteries.”
One reason why the SEI is not well understood is because it is difficult to observe during battery operation.
Now, Chen and his group members have used a state-of-the-art scanning transmission electron microscope to observe how the SEI evolves during charging and discharging in a specially designed liquid cell that closely mimics the conditions inside a battery.
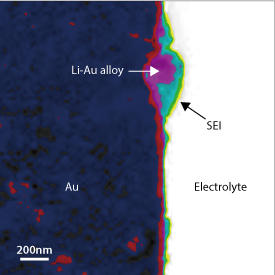
The researchers were able to image at a sub-nanometer resolution, which allowed them to see structures made up of inorganic and organic layers in the SEI (see image). “We were surprised that our scanning transmission electron microscope technique allowed us to clearly resolve the bilayer structures of the SEI,” explains Chen. These structures strongly suggest that the SEI grows through chemical reduction of the electrolyte at the boundary between the anode and the electrolyte. The team found that the growth of the SEI is also assisted by radical species that may form during the beginning stages of charging and discharging.
Chen and his co-workers also discovered that the SEI fails when it breaks and the inorganic layers come into contact with and rapidly dissolve in the electrolyte.
These findings will help researchers to improve lithium-ion batteries. “The microscopic insights we have obtained have important implications for understanding the kinetics of the SEI and for developing high-performance anodes that are protected by robust SEI films,” says Chen.
In the future, Chen’s team intends to further investigate the effect of electrolyte additives on the formation of robust SEI films.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.