

04/26/2019
© 2019 Shin-ichi Orimo
A new hydride developed by AIMR researchers could help finally realize all-solid-state lithium batteries1. This opens up a new direction for scientists to pursue in the race to develop better performing replacements for ubiquitous lithium-ion batteries.
Lithium-ion batteries are used extensively to power everything from smartphones to electric vehicles. But they can suffer from several drawbacks, including low energy densities, leakage of the liquid electrolyte, and the potential to burst into flames. All three problems could be overcome by replacing the liquid electrolyte with a solid one.
Lithium metal is the material of choice for the anodes of such all-solid-state batteries, but existing solid electrolytes undergo unwanted side reactions with lithium, which increase the resistance between the anode and electrolyte. This in turn degrades battery performance after repeated charging and discharging.
Complex hydrides are inorganic compounds that consist of a positive metal ion and a molecular anion that contains hydrogen. While they do not undergo side reactions with lithium, they suffer from a low lithium ion conductivity.
Now, Sangryun Kim of the Institute for Materials Research (IMR) at Tohoku University, together with IMR and AIMR colleagues, has developed a complex hydride that combines both a high lithium conductivity and a good stability with lithium metal anodes, such that they do not react with them.
“This is the first time that complex hydrides have been shown to be suitable solid electrolytes for practical lithium-metal batteries,” notes Kim. “To our surprise, we found that the resistance between our complex hydride and a lithium metal anode was almost negligible, meaning that lithium ions can freely move between the anode and electrolyte with scarcely any barrier.”
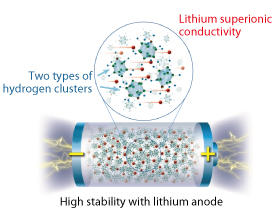
The researchers developed the complex hydride by tailoring the structures of its hydrogen clusters. The complex hydride consists of a mixture of two types of anions (see image): 70% of anions contain one carbon atom and nine boron atoms (as in (CB9H10)-), while 30% contain one carbon atom and eleven boron atoms (as in (CB11H12)-).
“This material is a totally new kind of solid electrolyte,” says Kim. “We hope our results will inspire future efforts to find lithium superionic conductors based on complex hydrides and also open up a new trend in solid electrolyte materials, which may lead to the development of high-energy-density electrochemical devices.”
The team intends to further improve their electrolyte. “There are many other complex anions that we can investigate,” comments Kim. “Taking the present work as the first step, we intend to conduct systematic studies to produce complex hydrides that have even better lithium ion conductivity and stability with lithium metal anodes.”
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.