

08/27/2018
© 2018 Katsumi Tanigaki
After seven years of systematic study, AIMR researchers have found that a family of organic materials boasts some surprising electronic properties1. Their findings could have implications for research into high-temperature superconductors.
Carbon-based molecules are increasingly being used in electronic components such as transistors. These organic materials can be very sensitive to light, or electric and magnetic fields, and it is relatively easy to fine-tune their properties by chemically modifying them.
Some of these materials are based on hydrocarbons known as polyacenes. Their structures consist of benzene molecules joined in a linear chain. The simplest one, naphthalene, contains two benzene rings; adding more rings makes anthracene, tetracene and pentacene. Other hydrocarbons, such as picene and phenanthrene, contain similar chains in a zigzag pattern.
Researchers had previously reported that when metals are added to hydrocarbons like these — a process known as doping — the materials can carry an electrical current with no resistance, a property called superconductivity. However, there is considerable debate about this behavior.
Katsumi Tanigaki of the AIMR at Tohoku University and colleagues investigated the electronic properties of a range of these doped molecules. Yet multiple tests of potassium phenanthrene and potassium picene uncovered no superconductivity, contradicting earlier findings.
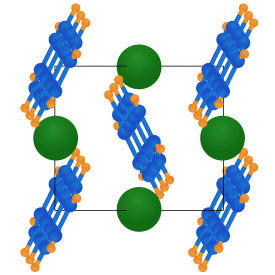
To further understand the behavior of these materials, the researchers developed a method for making doped polyacenes that produced higher quality crystals than had previously been available. X-ray diffraction measurements revealed that the potassium atoms in these compounds are trapped between flat planes of organic molecules (see image). This implies that the size of the metal atom will significantly affect the structure and properties of the crystal. Unlike potassium anthracene, for example, rubidium anthracene shows a form of magnetism, called paramagnetism, at room temperature.
Tanigaki’s team also found that all of the doped polyacenes they studied are electrical insulators under normal conditions. However, while potassium tetracene and potassium pentacene are classical insulators, potassium anthracene and potassium naphthalene are Mott insulators, meaning that they can become conductors under certain circumstances. For example, the team found that the electrical resistance of potassium anthracene gradually declined as they ramped up the pressure. “We’ve succeeded in measuring intrinsic electrical transport systematically in doped polyacenes for the first time,” notes Tanigaki.
The electrons in Mott insulators can interact in a similar way to how electrons behave in high-temperature superconductors, so these findings may offer valuable clues for further superconductivity studies. “Our studies of doped polyacenes from normal pressure to high pressure could provide important insights for future exploratory research of high-temperature superconductors,” says Tanigaki.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.