

10/30/2017
© 2017 Jiuhui Han
By using a state-of-the-art electron microscope, AIMR researchers have explored the inner workings of a lithium–oxygen battery1. The insights gained from these observations will facilitate the development of high-performance, next-generation batteries.
All batteries consist of an ionically conductive material (electrolyte) sandwiched between two electrodes. Lithium–oxygen batteries can potentially store more energy per battery weight than any other kind of battery, making them suitable as next-generation batteries for powering electric vehicles and storing electricity generated by renewable sources. But many hurdles need to be overcome before they can be used in practical applications.
Most of these problems stem from lithium–oxygen batteries’ high overpotential — the difference between the theoretical potential predicted by thermodynamics and that observed in actual experiments. One way around this deficit is to use a ‘redox mediator’, which transfers charge between the electrode and the lithium oxide that forms during discharging. However, a better understanding of the processes occurring inside batteries is needed to optimize this approach.
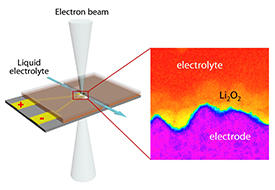
Now, Chuchu Yang, Jiuhui Han and Mingwei Chen of the AIMR at Tohoku University, along with co-workers, have used a state-of-the-art scanning transmission electron microscope to observe the dynamics of the reactions in a lithium–oxygen battery with a redox mediator and a liquid electrolyte as they occurred. The observation conditions were designed to imitate those under which actual batteries operate.
“In situ transmission electron microscopy has been used for years to study the electrochemical reactions of battery electrode materials,” notes Han. “However, previous microbatteries usually had solid-state electrolytes and were operated under vacuum and discharged and charged at far-from-equilibrium states. Consequently, they functioned very differently from actual batteries.”
To better emulate the conditions of conventional lithium–oxygen batteries, the team used a liquid cell that holds the liquid electrolyte and can be operated under ambient pressure (see image). In addition, they charged and discharged the microbattery in a similar manner to conventional battery testing. “The experimental setup mimics well the conditions of actual batteries and hence the phenomena observed in our study should translate well to real-world batteries,” says Han.
“Our findings have important implications for the fundamental understanding of lithium–oxygen electrochemistry,” says Han. “And they will promote the development of high-performance lithium–oxygen batteries as next-generation electrochemical energy-storage devices through inspiring design principles for electrodes and redox mediators.”
The team intends to use the findings of this study to develop advanced cathodes and redox mediators for high-performance lithium–oxygen batteries. They will also use their liquid-cell transmission electron microscopy technique to study other interesting chemical or electrochemical reactions in situ.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.