

08/29/2016
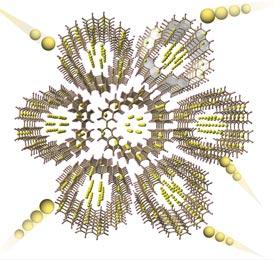
Figure courtesy of Takuji Ikeda, National Institute of Advanced Industrial Science and Technology (AIST).
A material that packs high levels of lithium ions into well-ordered nanochannels can open the door to all-solid-state batteries, reports a joint team from the AIMR1.
Rechargeable batteries, such as those used to power mobile phones, employ graphite as negative electrodes to store and release lithium ions. To meet consumer demand for longer-lived batteries, researchers are exploring different, nanostructured electrodes. In addition, the emergence of solid-state electrolytes, which are safer than conventional liquid electrolytes, is further driving the search for novel electrodes.
Now, Hiroyuki Isobe, Sota Sato and Shin-ichi Orimo from the AIMR at Tohoku University and colleagues have developed a nanostructured substance that can reversibly store and supply more lithium ions than conventional graphite electrodes. The team’s compound, known as [6]cyclo-2,7-napthylene (CNAP), is a flat, aromatic molecule. It is based on ultrathin graphene molecules but with a critical difference — it has an empty, central opening that spans almost a nanometer.
The Isobe group originally designed CNAP as a material for organic light-emitting diodes. They demonstrated bipolar charge transport with this compound but did not find any unique effects arising from its hole-like defect. Instead, through collaboration with the Orimo group, the team explored whether CNAP’s pore could be used for lithium-ion electrodes.
The researchers fabricated a solid-state battery by milling and then pressing a mixture of CNAP and the crystalline electrolyte lithium borohydride (LiBH4) together into a pellet. Supplying the composite with a lithium foil completed the cell and enabled the battery’s capabilities to be examined. The team saw a dramatic increase in lithium storage — nearly double the levels in graphite electrodes — that remained high even after multiple charge–discharge cycles.
Synchrotron X-ray analysis revealed that the doughnut-shaped CNAP stacked side-by-side to produce extended networks of one-dimensional, nanoscale pores; nearly 10 per cent of the electrode contained empty space to hold excess charge. Quantum mechanical models showed that this process is energetically favorable, with each CNAP able to capture up to 18 lithium ions.
Discovering that CNAP was compatible with solid-state electrolytes was a pleasing surprise for Sato and Isobe. “Naphthalene and LiBH4 are ubiquitous reagents that appear in any basic textbook for organic chemists,” they note. “But according to received wisdom, they are not expected to react together. This ‘no-reaction relation’ of our composite electrode was key to its quick, reversible operation.”
The team is currently exploring the design of functional pores by synthesizing new additions to their library of hydrocarbon-based cyclic molecules.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.