

07/25/2016
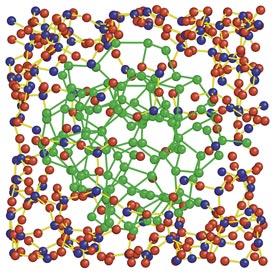
Reproduced from Ref. 1 andlicensed under CC BY 4.0 © 2016 A. Hirata etal.
AIMR researchers have used state-of-the-art experimentaltechniques and calculations to definitively determine the atomic structure of amorphoussilicon monoxide (SiO) for the first time1.
Lithium-ion batteries power everything from mobile devicesto electric vehicles, yet the race to improve their capacity continues. Silicon-basedanodes are promising in this pursuit, because silicon can hold more lithiumthan common carbon-based materials. However, the structure of crystallinesilicon deteriorates over charging cycles, reducing cell performance. Incontrast, amorphous (non-crystalline) silicon monoxide does not suffer fromsuch deterioration.
But despite having been extensively researched for nearly acentury, the atomic structure of amorphous silicon monoxide had not been fullydetermined, making it difficult to exploit the material's full potential.
“Amorphous silicon monoxide is a promising electrodematerial for lithium-ion batteries,” says Akihiko Hirata of the AIMR at Tohoku University.“But to understand its charge–discharge mechanism at the atomic level, it is vitalto know the details of its atomic structure.”
Hirata, Mingwei Chen and co-workers, along with collaborators at the National Institutefor Materials Science (NIMS) and Nissan, have succeeded in determining the atomicstructure of silicon monoxide by using an array of impressive experimentaltechniques and calculations.
They used angstrom-beam electron diffraction, a technique that Hirata and Chen had previouslydeveloped, to obtain diffraction datafrom nanoscale regions of the material. In combination with synchrotron X-rayscattering and computer simulation results, their investigation revealed thaton an atomic scale, silicon monoxide consists of a mixture of clusters ofsilicon and silicon dioxide with ‘sub-oxide’ regions in between (see image).
There had been much debate about the atomic structure ofamorphous silicon monoxide. Previous studies had suggested that it consisted ofan equilibrium mixture of amorphous silicon and silicon dioxide, but this modelcould not explain X-ray diffraction patterns. Conventional experimentaltechniques lack the spatial resolution to discover the atomic-scale structure,but angstrom-beam electron diffraction overcomes this restriction using anelectron beam smaller than 1 nanometer in diameter.
The team is excited about the potential of their method fordetermining local atomic structures in amorphous materials. “Heterogeneousamorphous materials such as silicon monoxide are important for next-generationenergy devices,” says Hirata. “Our method will enable more-reliable models to beobtained. It promises to be a powerful tool for understanding their atomicstructures as well as for providing valuable information about their functions.”
The team intends to use their technique to analyzeother amorphous oxides for batteries and chalcogenides for phase-change memorydevices.
Hirata, A.,Kohara, S., Asada, T., Arao, M., Yogi, C., Imai, H., Tan, Y., Fujita, T. &Chen, M. Atomic-scale disproportionation inamorphous silicon monoxide. Nature Communications 7, 11591 (2016). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.