

05/30/2016
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
By mapping the three-dimensional arrangement of gold atoms in gold structures containing nanoscale pores, researchers at AIMR have shown that surface defects play an important role in their catalytic ability1.
Gold nanostructures are widely used to accelerate oxidation reactions. For example, they can catalyze the oxidation of toxic carbon monoxide to benign carbon dioxide. But just how they speed up these reactions has been vigorously debated for many years.
One complicating factor is that gold nanoparticles are often supported on oxide surfaces, which makes it difficult to determine the degree to which substrate effects contribute to catalysis. Ideal systems for studying gold’s intrinsic catalysis are gold structures riddled with tiny pores ― nanoporous gold ― because, unlike nanoparticles, they do not need to be attached to a substrate.
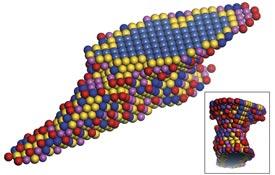
Now, Mingwei Chen and Pan Liu of the AIMR at Tohoku University and co-workers have combined a state-of-the-art electron microscopy technique with tomography to visualize in three dimensions the atomic arrangements of narrow bridges in nanoporous gold (see image). They then compared these three-dimensional maps with measurements of catalysis.
In addition to showing the positions of atoms, the images also reveal their degree of coordination to surrounding atoms. Atoms far from a surface are completely surrounded by atoms and hence have high coordination numbers, whereas those near or on a surface have lower ones. The researchers found qualitative agreement between the electron microscopy characterization and catalytic measurements at active atomic sites and low-coordinated surface atoms. This implies that defects on the surface play a major role in the catalysis of nanoporous gold.
“The curved surface of nanoporous gold is composed of flat terraces, atomic steps and kinks, which correspond to different coordination numbers,” explains Liu. “Generally, under-coordinated sites with low coordination numbers of five and six are thought to be highly active sites in oxidation reactions, whereas those with coordination numbers greater than nine are considered to be relatively inactive. Our study has confirmed this and provides compelling evidence that catalysis may originate from the under-coordinated surface atoms alone.”
Liu notes that the method could also be applied to other catalytic systems. “This study also demonstrates that quantitative measurements on an atomic scale can provide vital knowledge about the catalytic mechanisms of nanomaterials.”
In the future, the team intends to improve the catalytic performance of gold catalysts by fabricating and measuring the catalytic properties of nanoporous gold particles with larger surface areas and more under-coordinated atoms.
Liu, P., Guan, P. Hirata, A., Zhang, L., Chen, L., Wen, Y., Ding, Y., Fujita, T., Erlebacher, J. & Chen, M. Visualizing under-coordinated surface atoms on 3D nanoporous gold catalysts. Advanced Materials 28, 1753–1759 (2016). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.