

01/26/2015
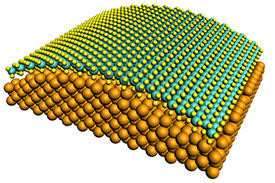
Modified, with permission, from Ref. 1 © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
AIMR researchers have devised a novel way to produce high-activity catalytic films for the electrocatalytic production of hydrogen, an important future energy storage medium. Their method potentially has a much broader application, as it could provide a new way to fabricate two-dimensional catalysts that possess both large effective surface areas and high catalytic activities.
Hydrogen offers an attractive, environmentally friendly way of storing energy as it can be produced by splitting water into its constituent elements through simply applying an electrical current in the presence of a catalyst ― a process known as the electrolysis of water. The problem with present methods of electrocatalytic hydrogen production is that most of them employ platinum-based catalysts, which are prohibitively expensive for practical applications.
Molybdenum disulphide (MoS2) is emerging as a promising cheaper alternative catalyst. As research has suggested that atoms located at the edges of MoS2 mainly contribute to the catalysis of hydrogen production, much effort has gone into producing catalysts with a high proportion of edges. But such catalysts can have reduced stability and electrical conductivity.
Now, Mingwei Chen and co-workers at the AIMR and other institutions in Japan and China have struck upon a totally different approach that enables them to obtain a high catalytic activity from an essentially ‘edge-free’ continuous MoS2 film1.
Their approach involves growing a single-molecule-thick film of MoS2 on a gold substrate riddled with tiny holes that are approximately 100 nanometers in diameter (see image). To their surprise, they obtained a catalytic efficiency for hydrogen production that rivals those of the best MoS2-based catalysts reported to date.
To explore the cause of this high activity, the researchers performed theoretical calculations. The results revealed that the bending of the monolayer MoS2 film induced by the puckered surface of the gold substrate altered the chemical properties of the film. The team considers that the out-of-plane strains induced by the curved topology are responsible for the enhanced catalytic activity of the MoS2 film.
The scientists are excited about this finding as it provides new insights into the effect of strain on the catalytic properties of two-dimensional catalysts and potentially could open up a new way to tailor the catalytic activity of two-dimensional catalysts through lattice strain engineering.
“This method allows us to effectively pack two-dimensional materials into three-dimensional devices,” states Chen, “while keeping the high accessible specific surface areas of two-dimensional films.”
The researchers are currently investigating cheaper substrates such as nickel and graphene as replacements for gold.
Tan, Y. W., Liu, P., Chen, L. Y., Cong, W. T., Ito, Y., Han, J. H., Guo, X. W., Tang, Z., Fujita, T., Hirata, A. & Chen, M. W. Monolayer MoS2 films supported by 3D nanoporous metals for high-efficiency electrocatalytic hydrogen production. Advanced Materials 26, 8023-8028 (2014). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.