

11/21/2014
© 2014 Patrick Han
An interdisciplinary team has devised a way to self-assemble defect-free graphene nanostructures right where they are needed by exploiting the reaction-directing properties of copper surfaces1. This is a significant advance since high-speed graphene transistors can outpace current silicon technology, but fabricating these atom-thin sheets of carbon into device structures had been problematic.
The team, which is led by Patrick Han and Taro Hitosugi from the AIMR at Tohoku University, is investigating experimental materials known as graphene nanoribbons (GNRs) for use in tiny transistor devices. The electronic states of these narrow graphene strips strongly depend on the atoms bordering their edges. A GNR is semiconducting when the edge carbons bond into an ‘armchair’ configuration, whereas a ‘zigzag’ configuration of edge carbons produces a metallic GNR with quantum confined spin states.
Because conventional circuit lithography inevitably introduces defects into GNRs, recent studies have explored a more direct, ‘bottom-up’ approach — growing graphene strips atom by atom by depositing molecular precursors onto metal surfaces. Typical strategies use chemically active precursors with relatively inert gold and silver substrates to give molecules many chances to interact with one another. But these methods have so far produced only armchair GNRs.
To synthesize zigzag nanoribbons, the researchers investigated whether a more reactive copper surface could direct a molecular polymerization reaction. “On surfaces like copper, molecules are less free to diffuse randomly and are more inclined to interact with an ordered lattice of metal atoms,” explains Han.
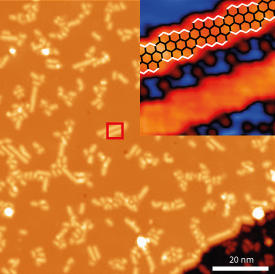
The researchers deposited precursors known as 10,10ʹ-dibromo-9,9ʹ-bianthryl monomers onto a copper surface and then used atom-resolved scanning tunneling microscopy to scrutinize the structures of samples produced at different annealing temperatures. They discovered that at room temperature the precursors self-assembled into narrow chains that preferentially grew in only six directions along the copper lattice. Stepwise heating to 500 degrees Celsius caused these chains to bunch into islands before joining up into periodic regions of zigzag nanoribbons (see image).
Further experiments revealed that the bromine atoms on the precursor helped hold the molecules into the zigzag arrangement at high temperatures until the graphene polymerization reaction occurred at 500 degrees Celsius. This is surprising since copper surfaces normally break off bromine atoms from aromatic molecules at room temperature.
The scientists anticipate that the fine control over nanoribbon length, direction and edge conformation this technique offers will spur more studies into surface-directed chemistry. “By exploiting the properties of a reactive surface, we can determine which nanoribbon structure will form and restrict its growth to specific directions,” says Han.
Han, P., Akagi, K., Canova, F. F., Mutoh, H., Shiraki, S., Iwaya, K., Weiss, P. S., Asao, N. & Hitosugi, T. Bottom-up graphene-nanoribbon fabrication reveals chiral edges and enantioselectivity. ACS Nano 8, 9181–9187 (2014). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.