

05/26/2014
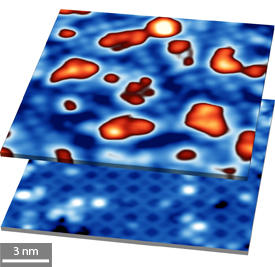
Modified, with permission, from Ref. 1 © 2014 American Chemical Society
Metal oxides play many important roles across a variety of high-tech energy applications, such as photovoltaic cells and lithium-ion batteries. Recently, scientists discovered that the electronic properties of extremely thin metal oxide films are unlike those of any other material. Developing metal oxide thin films into practical devices requires atomic-level knowledge of their structures and properties. Unfortunately, the complex chemical arrangement of such films makes them difficult to characterize using standard techniques.
Takeo Ohsawa, Taro Hitosugi and colleagues from the AIMR at Tohoku University have now used scanning tunneling microscopy to visualize atoms inside metal oxide thin films1. This approach provided the researchers with unprecedented surface images of strontium titanate (SrTiO3) — an insulating metal oxide that transforms into a two-dimensional conductor, a magnet or even a superconductor when interfaced with lanthanum aluminate (LaAlO3).
According to Ohsawa, many aspects of this LaAlO3/SrTiO3 system are controversial — particularly a phenomenon known as the ‘termination layer’ problem. SrTiO3 is made of alternating sheets of titanium dioxide (TiO2) and strontium oxide (SrO), which means that it exhibits contrasting effects, depending on which layer is in contact with LaAlO3. Films terminating with TiO2 become two-dimensional conductors, whereas those terminating with SrO retain their insulating properties.
To investigate this problem, the researchers combined a low-temperature scanning tunneling microscope with a laser-driven, thin-film deposition system. This allowed them to observe the early stages of SrO film growth — a critical period during the establishment of a new material. First, they prepared an ultra-clean SrTiO3 surface terminated by a periodically arranged layer of titanium and oxygen atoms. Then they used the scanning tunneling microscope to monitor how the surface changed as a pulsed laser slowly deposited increasing amounts of SrO.
Instead of forming crystalline structures, the SrO atoms clumped together into random, ‘island’-type arrangements (SrOx) that disrupted the periodicity of the underlying titanium–oxygen layer (see image). Careful comparisons of pre- and post-deposition surfaces revealed that titanium atoms merged with the SrOx islands during this growth phase, thus forming an atomic mixture that can disrupt interfacial electron transport.
“These results indicate the possibility of titanium atom incorporation into LaAlO3 films when they are deposited on SrO-terminated SrTiO3 substrates, which may lead to the suppression of two-dimensional conductivity in this system,” says Ohsawa. The researchers say that the need to account for excess titanium at the LaAlO3/SrTiO3 interface should help to solve the termination layer problem, while also giving researchers a blueprint for developing atom-by-atom engineered oxides with exotic electronic properties.
Ohsawa, T., Shimizu, R., Iwaya, K. & Hitosugi, T. Visualizing atomistic formation process of SrOx thin films on SrTiO3. ACS Nano 8, 2223–2229 (2014). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.