

04/25/2014
Foreground: Modified from Ref. 1 © 2014 American Chemical Society; background: © 2014 Takeshi Fujita
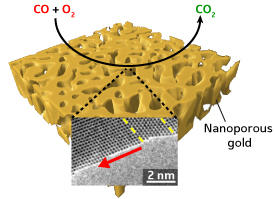
Nanoporous gold has a crucial role as a catalyst in the production and processing of commodity chemicals under environment-friendly conditions. Its tiny pores provide exceptional activity in reactions that involve oxygen, such as the thermodynamically challenging transformation of carbon monoxide (CO) into carbon dioxide. However, the mechanism that governs its degradation and loss of activity during catalysis has so far remained elusive.
By using sophisticated microscopy techniques to monitor changes in the structure of nanoporous gold during CO oxidation, a team led by Takeshi Fujita from the AIMR at Tohoku University has gained unprecedented experimental insight into the catalysis-induced degradation mechanism at the atomic scale1. Their work has also revealed the significance of planar defects — and in particular the formation of twin boundaries — in averting this process, thereby offering new avenues for enhancing catalytic activity and stability.
The researchers first synthesized freestanding nanoporous gold leaves through a dealloying process, in which nitric acid removes the silver from an ultrathin gold–silver sheet. The resulting gold structure comprised nanopores embedded in flat, close-packed ‘terraces’ separated by single-atom ‘steps’.
The nanoporous gold catalyst did not exhibit any noticeable change under electron beam irradiation in pure CO or oxygen environments or under vacuum, indicating its stability. Upon exposure to a CO–air gas mixture, the nanopores and connecting material expanded with increasing reaction time, which was associated with a reduction in catalytic activity. Furthermore, silver and gold in the connecting material were initially uniformly distributed but gradually became separated as the reaction progressed.
To gain insight into this coarsening mechanism, Fujita and colleagues used an environmental transmission electron microscope to obtain high-resolution images of individual nanopores under reaction conditions. When they increased the reaction time, the nearly round nanopores became faceted and widened before merging with neighboring pores.
The researchers found that the coarsening relied on the rapid oxidation-induced migration of gold atoms on the single-atom reactive steps of the uppermost terrace (see image). The nanopores grew fastest perpendicular to the twin interfaces — a clear indication of the importance of these defects. In the absence of twin defects, the nanopores did not display any preference in growth direction. “Twin planes can effectively pin the gold atoms to the surface, eventually suppressing nanopore coarsening,” explains Fujita.
The team is currently evaluating ways to boost catalyst performance and longevity by augmenting the density of planar defects in nanoporous gold. “We are also trying to develop new exhaust gas catalysts based on this system,” adds Fujita.
Fujita, T., Tokunaga, T., Zhang, L., Li, D., Chen, L., Arai, S., Yamamoto, Y., Hirata, A., Tanaka, N., Ding, Y. & Chen, M. Atomic observation of catalysis-induced nanopore coarsening of nanoporous gold. Nano Letters 14, 1172–1177 (2014). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.