

02/24/2014
Modified, with permission, from Ref. 1 © 2013 American Chemical Society
The plastic materials used in organic solar cells are abundant and cheap, making such devices promising for harvesting solar energy. The complexity of the organic molecules used in these cells, however, has prevented a full understanding of the mechanism by which they convert sunlight into electrical charge. This complexity has also hampered their use in practical applications. Now, Hiroyuki Tamura and Irene Burghardt from the AIMR at Tohoku University and the Goethe University Frankfurt, Germany, respectively, have shown how the distribution of electrons across an organic solar cell plays a crucial role in generating electricity1.
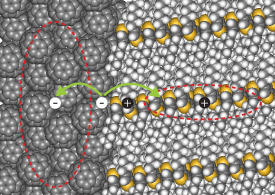
Organic solar cells are typically comprised of a mixture of polymer molecules and cage-like spherical balls of carbon known as ‘buckyballs’. Light incident at the interface between these molecules excites the electrons present in the molecules. As the electrons move across the molecules, they leave behind empty spaces — ‘holes’ — which are also mobile. The role of the buckyballs is to transport the electrons away to the contact electrode while the polymers carry the corresponding holes (see image).
A crucial aspect for the operation of an organic solar cell is the initial charge separation of electrons from the polymer into the buckyballs. “A faster charge separation leads to a more efficient operation of the solar cell as it minimizes the opportunity for electrical losses,” explains Tamura. However, the size and complexity of the organic molecules involved has impeded a deeper understanding of this process.
To investigate the charge-transfer mechanism, Tamura and Burghardt used powerful quantum dynamical simulations that were able to compute the properties of large systems. Their results reveal that the charge transfer is not initiated by the simple ‘hopping’ process of electrons from the polymers to the buckyballs.
Rather, the charge transfer relies on the fact that in quantum physics each electron can also be described as a wave, which is spread out in space. When an electron is excited by sunlight, its wavefunction expands. This expansion lowers the energy barrier for the charge-transfer process to occur, thus enabling the electrons to move to the buckyballs. “Unlike hopping processes, this scheme can explain the efficient, ultrafast charge-separation observed in some experiments,” explains Tamura.
The new mechanism will be important for the design of enhanced organic solar cells. For example, adds Tamura, “the reduced energy barrier will allow solar cell designs that enable the absorption of a broader cross-section of sunlight, especially at longer wavelengths.”
Tamura, H. & Burghardt, I. Ultrafast charge separation in organic photovoltaics enhanced by charge delocalization and vibronically hot exciton dissociation. Journal of the American Chemical Society 135, 16364−16367 (2013). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.