

02/24/2014
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Cultured cells can form functional, three-dimensional tissue if given a suitable scaffold to guide their growth. Biocompatible hydrogels — which contain mostly water — provide useful scaffolds for some cell types. However, the usual method of stiffening hydrogels by reducing their water content can impede cell growth. In addition, hydrogels do not typically conduct electricity, making them unsuitable for cells that require electrical stimulation to grow.
Samad Ahadian and colleagues from the AIMR at Tohoku University have now used carbon nanotubes to create a hydrogel that is both sturdy and a good conductor of electricity1. The addition of nanotubes presents an alternative way to tune both the mechanical and electrical properties of hydrogels, the researchers say.
Using a gelatin methacrylate hydrogel as a base, the researchers added multi-walled carbon nanotubes — thin straws of carbon atoms measuring 40–90 nanometers in diameter — to double the hydrogel’s stiffness.
Initially, the nanotubes were randomly oriented in the gel, but the researchers were able to align them by applying an electric field. Next, they used a burst of ultraviolet light to form bonds between the gelatin methacrylate molecules, fixing the nanotubes in place. The nanotube network enabled the gel to carry between 100 and 1,000 times more current than the unaligned nanotubes, depending on the nanotube concentration.
The researchers then tested their hydrogel as a growth medium for C2C12 mouse muscle myoblasts, a type of muscle cell that can fuse together to form myotubes. The myotubes in turn mature into myofibers, which behave like normal muscle tissue.
More than 95 per cent of the myoblasts seeded into the nanotube-laden gel survived. Additionally, after three days in culture the growth of the cells accelerated thanks to the greater number of anchoring sites offered by the nanotubes.
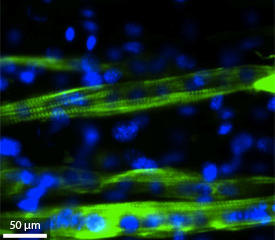
By creating a groove in the hydrogel, the researchers were able to encourage myoblasts to line up end-to-end, helping them to form healthy myotubes that could contract properly. After eight days in culture, the researchers applied a small voltage that promoted muscle cell differentiation along the groove — an effect that was most pronounced in the aligned-nanotube hydrogel (see image).
Hydrogel-grown muscle tissue has numerous potential medical applications. “Engineered muscle tissues can be used as an efficient platform to investigate drug candidates to treat diabetes,” explains Ahadian. “Such tissues can be made using a patient’s own muscle cells, which could be a major step toward personalized medicine and would make it possible to eliminate expensive and time-consuming animal experiments for drug screening.”
Ramón-Azcón, J., Ahadian, S., Estili, M., Liang, X., Ostrovidov, S., Kaji, H., Shiku, H., Ramalingam, M., Nakajima, K., Sakka, Y. et al. Dielectrophoretically aligned carbon nanotubes to control electrical and mechanical properties of hydrogels to fabricate contractile muscle myofibers. Advanced Materials 25, 4028–4034 (2013). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.