

05/27/2013
© 2013 Jiri Orava
Phase-change materials are of great importance in modern computing due to their use in rewritable optical disks and computer memory. They are particularly suitable for these applications as they can be made to undergo a fast, reversible transition induced by light or electrical pulses. These transitions cause their molecular structure to change from an ordered crystal to an amorphous state resembling that of glass. Materials in these two states have vastly different physical properties, such as their ability to reflect light or conduct electricity, and this phenomenon can be used to encode the ‘on’ and ‘off’ states of digital bits.
An international team of researchers, including Jiri Orava from the AIMR at Tohoku University, has now discovered a completely new type of phase transition in which the material switches between two different amorphous states1. The amorphous-to-amorphous transition is fundamentally different to known amorphous-to-crystalline transitions, and even to previously known amorphous–amorphous transitions, according to Orava.
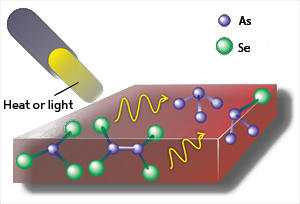
The researchers studied As50Se50, which is composed of equal amounts of arsenic (As) and selenium (Se). When freshly deposited as a thin film, the material is amorphous but if heated to high temperatures and slowly cooled it becomes crystalline. However, the team discovered that when only moderately heated, As50Se50 transitions to a different — and stable — amorphous state. Similarly, when irradiated by laser light, the newly grown material forms another stable amorphous state. These two states can be distinguished by measuring the bonding energy between the arsenic and selenium atoms, which provides a unique fingerprint for the atomic configuration.
Transitions between different amorphous states are not unknown, but previously had only been observed in materials subjected to pressure. The change studied here can be induced through heating, suggesting that a further mechanism is responsible for these amorphous-to-amorphous transitions. This mechanism is of particular interest because the material can be reversibly switched between the two amorphous states through successive annealing and laser-exposure cycles. Switching is thought to occur at milli- to microsecond intervals.
Reversible switching between amorphous states resembles previously discovered amorphous-to-crystalline transitions used in other applications. However, the new system offers the advantage that the switching process requires far less energy. “The changes in atomic arrangement take place with a relatively low cost of energy; no melting is required,” comments Orava. Moreover, the new switching paradigm involves far fewer atoms than switching to an ordered crystal, suggesting it could be used to create computer memory devices. Verifying the dynamics of this transition and its benefit for applications remains an aim of future research, says Orava.
Kalyva, M., Orava, J., Siokou, A., Pavlista, M., Wagner, T. & Yannopoulos, S. N. Reversible amorphous-to-amorphous transitions in chalcogenide films: Correlating changes in structure and optical properties. Advanced Functional Materials 23, 2052–2059 (2012). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.