

01/28/2013
© 2012 Tienan Jin
Nanoporous gold catalysts have recently gained popularity due to their long lifetime and green credentials. As heterogeneous catalysts — in the solid state and dispersed throughout the reaction mixture — they are convenient to handle, in contrast to homogeneous ones which are dissolved within the reaction medium. They have proved to be efficient in highly selective oxidation reactions, such as the conversion of an alcohol group (consisting of a C–OH moiety with a single carbon–oxygen bond) to a carbonyl group (a double-bonded carbon–oxygen moiety). Until recently, however, nanoporous gold was thought to be inactive for reductive hydrogenation reactions.
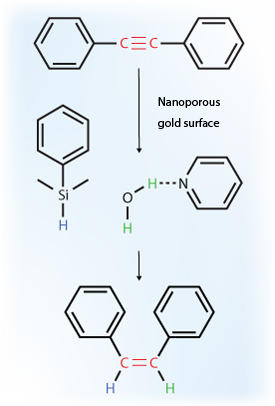
Tienan Jin and co-workers from the AIMR at Tohoku University, in collaboration with researchers from Japan and China, have now shown that this catalyst can be used in the selective hydrogenation of alkynes to alkenes, where carbon–carbon triple bonds are reduced to double bonds (see image)1. Interestingly, the reaction is both chemoselective and Z-selective. This means that in addition to it not progressing all the way to a single bond, the two hydrogen atoms added to the alkyne moiety are always placed on the same side of the bond, forming what is known as a Z-alkene. “The accomplishment of perfect Z-selectivity and chemoselectivity has previously been unsuccessful in both homogeneous and heterogeneous catalytic processes,” says Jin.
By replacing homogeneous palladium and platinum catalysts with the heterogeneous nanoporous gold, it has been possible to obtain higher yields of the desired alkenes. “Heterogeneous catalysts are also more robust than their homogeneous analogues and have a longer lifetime,” explains Jin. “Additionally, they can be easily recovered by filtration and reused several times.”
To produce the catalyst, a thin gold–silver film, featuring three-dimensionally curved nanopores, was subjected to electrochemical dealloying. Control experiments using deuterium-labeled species revealed that organosilane and water, the hydrogen sources in the reaction, each contributed a hydrogen. An appropriate amine additive, such as pyridine, was also required to prevent the formation of molecular hydrogen.
Jin’s team tested the nanoporous gold-catalyzed reaction on numerous alkynes, with both terminal (at the end of the molecule) and internal triple bonds. All showed complete conversion and almost all provided yields of above 90%.
“It is expected that nanoporous gold will not only be used in the selective reduction of various functional groups, but will also open opportunities for applications in more complicated heterogeneous catalytic methodologies for clean chemical synthesis,” comments Jin. “In preliminary experiments, we have already succeeded in the application of the present catalyst systems to the selective hydrogenation of heteroaromatic compounds and carbonyl moiety.”
Yan, M., Jin, T., Ishikawa, Y., Minato, T., Fujita, T., Chen, L.-Y., Bao, M., Asao, N., Chen, M-W. & Yamamoto, Y. Nanoporous gold catalyst for highly selective semihydrogenation of alkynes: remarkable effect of amine additives. Journal of the American Chemical Society 134, 17536–17542 (2012). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.