

10/29/2012
© 2012 J.-Q. Wang
Synthetic dyes with long-lasting, vivid colors are valuable to both manufacturers and consumers. However, these attributes also make it challenging to clean up these compounds when they slip into wastewater systems and become pollutants. ‘Azo’ dyes, for example, owe their colors to nitrogen double bonds that resist degradation from bacterial or carbon sorption treatments. Now, Jun-Qiang Wang from the AIMR at Tohoku University1 and co-workers in Japan and the US have described a way to degrade azo dyes with far greater efficiency than current techniques, using amorphous magnesium–zinc metallic glass powders.
One of the best ways to decompose azo dyes is by using pure, ‘zerovalent’ metals — in an oxidation state of 0 — such as magnesium or iron to transfer electrons to nitrogen double bonds and crack them apart. However, although zerovalent magnesium is particularly effective at removing organic contaminants from water, it has poor corrosion resistance and is eventually consumed by the aqueous environment. Materials researchers are thus focusing on finding low-cost methods to reduce the corrosion of magnesium while retaining its high reaction efficiency.
Alloying magnesium with another metal can enhance its resistance to corrosion, but only if the atoms are mixed together well. To achieve this, Wang and his co-workers melted magnesium and zinc atoms in a crucible, and then quickly cast them into thin ribbons of metallic glass. The metal atoms pack tightly and homogenously in this glassy state, giving the alloy excellent durability.
Then, to convert the magnesium-zinc ribbons into a form suitable for treating wastewater, the team pulverized them into a fine powder — an unconventional approach that has many advantages, according to Wang. “Powders have much larger surface areas compared to ribbons, which enhances their reactivity,” he says. “Also, the powdered form may be injected underground to deal with pollutants in groundwater.”
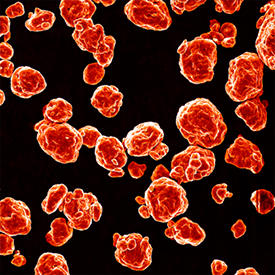
Microscopy experiments revealed that the magnesium–zinc powder was well dispersed and did not form aggregates (see image), making it ideal for reacting with synthetic contaminants in an aqueous solution. The powder de-colored a solution of azo dye within 10 minutes — clear evidence that the metallic glass completely degraded stubborn nitrogen double bonds.
Wang notes that in addition to a striking improvement in corrosion resistance, which the researchers attribute to the alloy’s amorphous atomic structure, magnesium–zinc metallic glass powders can decontaminate azo dyes with reaction efficiencies 1,000 times greater than that of commercial iron powders. “These materials can efficiently degrade organic water contaminants in harsh, anaerobic environments at a cheap price, opening up a broad field for metallic glass applications.”
Wang, J.-Q., Liu, Y.-H., Chen, M.-W., Louzguine-Luzgin, D. V., Inoue, A. & Perepezko, J. H. Excellent capability in degrading azo dyes by MgZn-based metallic glass powders. Scientific Reports 2, 418. | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.