

09/24/2012
© 2012 Nature Publishing Group
Twenty-five years ago, nobody thought that gold would have catalytic activity towards molecular oxygen, because classical thermodynamic principles reject any chemical reaction between gold and oxygen. But the discovery that gold particles smaller than five nanometers could oxidize carbon monoxide (CO) into carbon dioxide (CO2) at room temperature set off flurries of investigation. Soon, researchers had harnessed this size-dependent catalysis for applications such as decontaminating hydrogen gas in polymer fuel cells. The underlying mechanism for this catalytic activity however remains unclear.
Mingwei Chen, Takeshi Fujita, and co-workers from the AIMR at Tohoku University, in collaboration with researchers from Japan, the US, the UK and China, have captured new evidence that small defects on gold surfaces are active sites for CO oxidation reactions1. By developing state-of-the-art techniques to watch surface atomic structures evolve as catalysis occurs, the team also discovered that impurity atoms play critical roles in stabilizing the defect sites — a finding that could boost the longevity and activity of gold catalysts.
Most nanoparticles require an oxide support to hold them in place, making it difficult to pinpoint specific gold catalytic mechanisms. Chen and his team overcame this problem by studying a substance known as nanoporous gold. Produced by electrochemical de-alloying of a thin gold–silver precursor film, it has a three-dimensional, free-standing architecture of curved nanopores, making it ideal to study gold catalysis without interference from other materials.
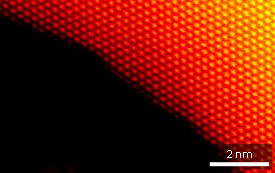
The researchers turned to spherical-aberration-corrected scanning transmission electron microscopy (Cs-corrected STEM) to identify catalytically active nanoporous gold surface structures. This technique suppresses lattice distortion effects caused by projection lenses, providing sub-atomic resolution capabilities. By carefully controlling gas pressures within a specially-designed specimen holder, the team performed in situ characterizations during a CO oxidation reaction in an environmental TEM. “Cs-corrected STEM gives us a great chance to see the true atomic structure of complex materials,” says Chen.
The team’s high-resolution images revealed that nanoporous gold’s surface structure consists of flat, close-packed terraces separated by single-atom steps (see image). Along bent portions of the nanopores, these steps fall out of alignment and become ‘kinks’ of under-coordinated gold atoms — exceedingly active sites for chemical oxidation. When the researchers exposed the nanoporous gold to a CO/air gas mixture, they observed that the terrace edges dynamically reconstructed into kinked arrangements, a clear sign that these defects play critical catalytic roles. Further experiments revealed that residual silver impurities in nanoporous gold preserved the high density of kinked defects, ensuring high catalytic performance.
Chen notes: “Although many theories have been put forward before, the story of gold catalysis is simpler than anyone ever thought.”
Fujita, T., Guan, P., McKenna, K., Lang, X., Hirata, A., Zhang, L., Tokunaga, T., Arai, S., Yamamoto, Y., Tanaka, N. et al. Atomic origins of the high catalytic activity of nanoporous gold. Nature Materials 11, 775–780. | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.