

11/28/2011
© 2011 ACS
The combination of two similar objects has the potential to create a completely different material. In recent years, the heterostructures of transition metal oxides have demonstrated a range of surprising properties, such as the interface between lanthanum aluminate and strontium titanate. Both materials are insulators in their bulk form, yet also show very high conductivity and under certain conditions can even become superconductive. Given the wide range of compositions these oxides can span, it is expected that more functionalities can be discovered — and possibly used in devices — through judicious combinations. But to achieve this, it is essential to understand and control the morphology and electronic structure of the interfaces at the atomic scale. So far, however, interface formation has been mainly studied at the unit-cell level.
Ryota Shimizu, Taro Hitosugi and colleagues at the WPI Advanced Institute for Materials Research at Tohoku University and other institutions in Japan have now zoned in on atom-by-atom growth through their construction of a high-resolution scanning tunneling microscopy combined with a pulsed laser deposition system1. This device enabled the team to closely investigate the formation of very thin strontium titanate films at atomic resolution2.
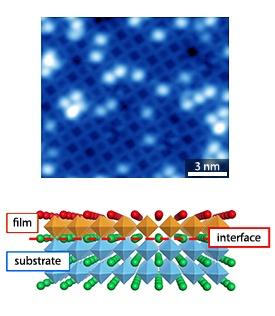
The researchers began their investigation with the homo-epitaxial atom-by-atom growth process of a perovskite material, strontium titanate. Using pulsed laser deposition, they grew a strontium titanate film on a substrate of the same material that has a specific perfectly ordered structure. “We found that this specific surface can be prepared in a wide range of oxygen partial pressures in a reproducible manner. This is why strontium titanate is an ideal substrate to serve as a model, which will then enable us to monitor at the atomic level the growth processes of perovskite oxides more generally,” explains Shimizu.
After the films were grown, the team observed the formation of island terraces with an atomic structure identical to that of the substrate. In particular, the results showed atomic-level coherency between the substrate and the thin film, whereby the substrate imposed its morphology on the newly grown film.
The results offer potential growth for other materials as well. “Our team is now conducting growths of other oxide materials, such as strontium oxide, lanthanum aluminate and manganite, to unveil the growth process and interfacial formation between different materials at the atomic scale,” says Shimizu. “These investigations may lead to the preparation of new heterostructures, or high quality thin films with exotic multifunctionality.”
Iwaya, K., Shimizu, R., Hashizume, T. & Hitosugi, T. Systematic analyses of vibration noise of a vibration isolation system for high-resolution scanning tunneling microscopes. Review of Scientific Instruments 82, 083702 (2011).
Shimizu, R., Iwaya, K., Ohsawa, T., Shiraki, S., Hasegawa, T., Hashizume, T. & Hitosugi, T. Atomic-scale visualization of initial growth of homoepitaxial SrTiO3 thin film on atomically ordered substrate. ACS Nano 5, 7967 (2011). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.