

04/25/2011
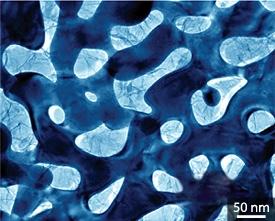
From Ref. 1 © 2011 M. Chen
Wind turbines, solar cells and other ‘green’ energy technologies often encounter supply-and-demand problems because their energy sources are intermittent. One way to overcome this is by using supercapacitors — devices that can quickly store electricity by organizing positively and negatively charged ions into two distinct electrolyte layers, each in contact with a metal electrode. However, while such double-layer supercapacitors are extremely robust, the energy storage density they offer is not sufficiently high for widespread use.
Xingyou Lang, Akihiko Hirata, Takeshi Fujita and Mingwei Chen from the WPI-AIMR have been working on building supercapacitors using transition metal compounds such as manganese dioxide (MnO2), which can store charge at metal sites by an electron transfer process called ‘pseudocapacitance’. Unfortunately, MnO2 has low conductivity, which limits its charging and discharging speeds. The researchers have now shown1 that a supercapacitor constructed using an MnO2-plated gold film dotted with nanoscale pores has quick-charging properties and unprecedented electrical storage capabilities.
Previous attempts to resolve the conductivity problems of MnO2 have involved incorporating the oxide into conductive polymers or carbon nanotubes. Chen and his team took a different approach by fabricating a nanostructured MnO2–gold composite. First, they selectively etched a silver–gold alloy into a thin gold sheet permeated with numerous nanopores. They then grew MnO2 nanocrystals directly into the pore channels using a gas-phase reaction. This growth step proved crucial to the performance of the supercapacitor — too much crystal deposition would fill the nanopores and impair the double-layer effect, while too little crystal growth would not provide good pseudocapacitance. Finally, the team sealed the resulting nanostructured electrodes and an aqueous electrolyte in plastic, creating a thin and flexible supercapacitor.
The device displayed excellent charge storage capacity with an energy density up to 20 times higher than that of other MnO2 electrodes. The supercapacitor also displayed near-ideal high-speed charging behavior, which high-resolution microscopy revealed to be due to intimate contact between the MnO2 crystals and the conductive gold surface (pictured). “We did not expect the formation of such a good interface because of the obvious differences in lattices and chemical properties between gold and MnO2,” says Chen.
These properties, in combination with fast ion diffusion through the three-dimensional nanoporous structure, result in an enhanced supercapacitor with promising applications. Chen and his team are currently investigating how to utilize the MnO2–gold composite for electrodes in lithium-ion batteries, and are attempting to develop other mixed nanoporous materials with even higher energy densities.
Lang, X., Hirata, A., Fujita, T. & Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nature Nanotechnology 6, 232–236 (2011). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.