

06/28/2010
© 2010 D. Hojo
Innovative technologies that use molecular-based ‘inks’ to print electronic circuitry onto flexible plastic substrates promise to usher in an era of futuristic low-cost devices, such as smart clothing tags that can communicate instructions to washing machines. Manufacturing printable electronics remains challenging, however, because the high temperatures usually needed to bind the inks to surfaces can damage the polymer materials used as substrates.
Researchers led by Daisuke Hojo and Tadafumi Adschiri from the WPI-AIMR at Tohoku University have now developed a simple and efficient way to attach an ink made of metal oxide nanocrystals onto surfaces at room temperature1. According to Hojo and Adschiri, the diverse conditions needed to deposit extremely small particles in an organized manner made this breakthrough a tough one to achieve. “For printable electronics, the nanoparticles have to be well-dispersed in solvents, but they also need to self-assemble into films during the drying stage, and to subsequently become immobilized on the substrate,” says Hojo. “For each step, the required interactions between the nanoparticles, the solvent, and the surface are different — making the fabrication process difficult,” says Adschiri.
Researchers at Tohoku University led by Adschiri had previously been successful in coating tiny particles of cerium oxide — a rare-earth compound with catalytic and optical capabilities — with a thin film of organic molecules to prepare highly soluble nanocrystals2. Using a process known as ligand exchange, the researchers demonstrated in their most recent study that cerium oxide nanocrystals can be immobilized on a silica surface by replacing one molecule bound to the nanocrystal with one bound to the substrate — achieving the effect of a chemical ‘glue’.
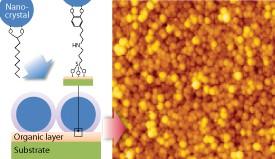
The scientists prepared the metal oxide nanocrystals for the process by coating the particles with decanoic acid, a long carboxylic acid that binds to cerium oxide via two oxygen atoms (Fig. 1). They then deposited the nanocrystals from solution at room temperature onto a silicon surface that had been coated with a thin organic layer of DHCA — a hydrocarbon bearing a catechol group (a benzene ring with two neighboring alcohol units). Chemical equilibrium effects drove the nanocrystals to swap the decanoic acid for the more favorable catechol groups fixed on the silica surface (see figure), effectively binding the nanocrystals to the substrate.
The researchers confirmed that this method produced dense films of surface-bound nanocrystals that were thermally stable up to 200 °C. They also found that other carboxylic acid-coated nanocrystals, including crystals with iron and titanium cores, could be trapped using the same technique because of the broad affinity of catechol groups. According to Hojo and Adschiri, this ligand-exchange method helps solve the critical problem of immobilizing nanoparticles. “This is a definite step toward establishing technology for printed electronics, especially for fabricating photovoltaic devices,” says Adschiri.
Hojo, D., Togashi, T., Iwasa, D., Arita, T., Minami, K., Takami, S. & Adschiri, T. Fabrication of two-dimensional structures of metal oxide nanocrystals using Si substrate modified with 3,4-dihydroxyhydrocinnamic acid. Chemistry of Materials 22, 1862–1869 (2010). | article
Zhang, J., Ohara, S., Umetsu, M., Naka, T., Hatakeyama, Y. & Adschiri, T. Colloidal ceria nanocrystals: A tailor-made crystal morphology in supercritical water. Advanced Materials 19, 203–206 (2007). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.