

04/26/2010
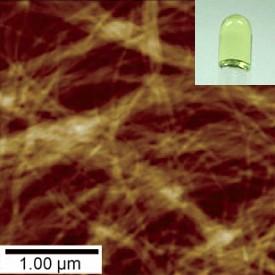
© 2010 Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
Ever since the realization that biomolecules such as DNA naturally assemble into three-dimensional helix shapes, researchers have tried to replicate these structures using different chemical units. Helicenes — spiral-shaped molecules made up of several fused benzene rings — have attracted much scientific interest because their conjugated framework leads to unique optical, electronic and aggregation properties.
Now, Masahiko Yamaguchi from the Advanced Institute for Materials Research at Tohoku University1 and colleagues have used short chains of helicenes, known as oligomers, to produce thermally reversible organic gels. The methodology developed by the research team promises to improve control over the properties of these hybrid liquid–solid materials at the molecular level.
When helicenes are synthesized, they form one of two chiral enantiomers — isomers of the same compound but arranged such that they are mirror images of one another. Most reactions produce equal amounts of both enantiomers, but these racemic mixtures usually form crystals with well-ordered lattices, not gels. In pure solutions of enantiomers, however, the tight packing needed for crystallization is perturbed, and the helicenes tend to aggregate into long fibers that trap solvent molecules in a gel.
Yamaguchi and his team developed a new series of ‘pseudoenantiomers’, which display better gel-forming properties than pure solutions. Composed of two helicene enantiomers with slightly different sizes, these substances spontaneously formed a yellow gel when mixed together at room temperature (Fig. 1). Furthermore, this process was thermally reversible: heating to 110 °C caused the gel to liquefy, but the gel re-formed on cooling to 25 °C. Yamaguchi says that the pseudoenantiomers take on properties that are intermediate between those of the pure enantiomers and the racemic mixture, suppressing crystallization and enhancing fiber formation.
The need for soft gel-based materials is expanding in diverse areas ranging from bioengineering to consumer packaging. Gels are suitable for these applications because of their ability to mimic the networks found in living systems and present properties distinct from those of conventional materials. Yamaguchi’s pseudoenantiomer method has the potential to improve gel compounds by providing a reliable means of joining small molecules together.
“Precisely constructing gels with specific functions, such as hardness, elasticity, flexibility or processability, requires a delicate method to control the gel structure,” says Yamaguchi. “In that sense, gel formation by small molecule aggregation is interesting because the structure of small molecules can be readily controlled, and a diversity of gels can be provided.”
The use of two-component gels offers great promise in terms of tuning properties, as different enantiomers ratios produce different gels. It also has other implications, according to Yamaguchi: “For example, one compound can be on a surface, and the other in solution. Such a system can provide control of the interface structure between the solid and liquid.”
Amemiya, R., Mizutani, M. & Yamaguchi, M. Two-component gel formation by pseudoenantiomeric ethynylhelicene oligomers. Angewandte Chemie International Edition 49, 1995–1999 (2010). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.