

Researchers have made a significant breakthrough in the development of catalysts for the electrochemical nitrate reduction reaction (eNO3RR) to ammonia, a process that has broad implications for sustainable energy, agriculture, and industrial applications.
Ammonia, a critical component in global food production, also holds promise as a zero-carbon fuel due to its high energy density, clean combustion products, and established infrastructure for storage and transportation. However, the current method of producing ammonia, the Haber-Bosch process, is energy-intensive and accounts for about 1.8% of global CO2 emissions.
In their recent study, the research team focused on spinel cobalt oxides (Co3O4), a promising class of catalysts for eNO3RR due to their low cost, high activity, and selectivity. The team synthesized various Co3O4 nanostructures with different crystallographic facets—{100}, {111}, {110}, and {112}—to investigate how these facets influence the catalyst's performance in ammonia production. The study revealed that the {111} facet of Co3O4 exhibited superior performance, achieving an impressive ammonia Faradaic efficiency of 99.1% and a yield rate of 35.2 mg h−1 cm−2.
“Our findings show that the {111} facet of Co3O4 is effective in transforming nitrate to ammonia,” said Dr. Heng Liu, the co-first author of the paper and a Specially Appointed Assistant Professor at the Advanced Institute for Materials Research (WPI-AIMR), Tohoku University. “This is due to the rapid formation of oxygen vacancies and Co(OH)2 on this facet, which significantly enhances the catalyst's performance.”
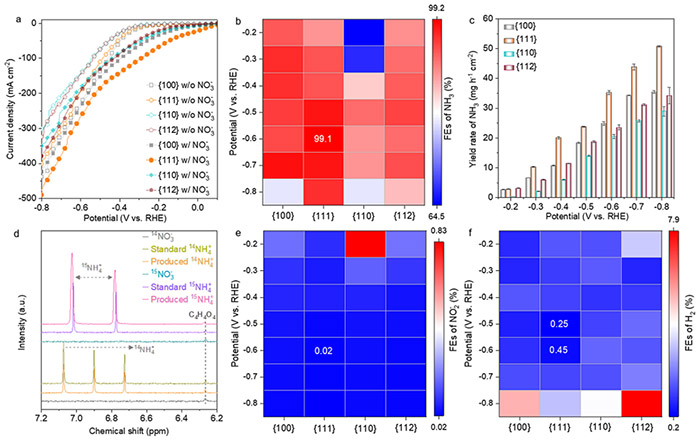
eNO3RR performance of Co3O4 {100}, {111}, {110}, and {112} catalysts. ©Hao Li et al.
In addition, the researchers discovered that the catalyst went through a transformation process during the reaction, evolving from Co3O4 to a structure with oxygen vacancies, then to a Co3O4−x-Ov/Co(OH)2 hybrid, and finally stabilizing as Co(OH)2. This process was most pronounced on the {111} facet, contributing to its superior performance.
“The structural changes we observed are crucial for understanding the catalyst's activity,” added Professor Hao Li, corresponding author of the paper and an associate professor at WPI-AIMR. “These insights will help us design more efficient catalysts by optimizing the exposed facets.”
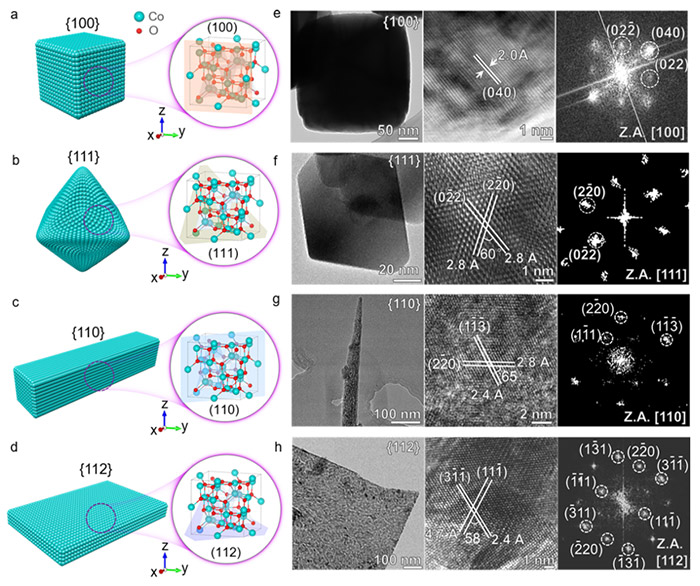
Structural characterization of Co3O4 nanostructure with different facets. ©Hao Li et al.
Ammonia’s importance extends beyond agriculture, as it is a potential zero-carbon fuel and a key player in energy conversion and storage technologies. The eNO3RR offers a sustainable alternative to the Haber-Bosch process, transforming nitrate waste into valuable ammonia while aiding environmental remediation.
“This research lays a solid foundation for the development of more efficient, sustainable catalysts,” states Li. “As we move forward, our goal is to control the final phases of the catalyst’s transformation to further enhance its activity, selectivity, and stability.”
This breakthrough in understanding and optimizing Co3O4 catalysts could pave the way for cleaner and more sustainable industrial processes, contributing to the global efforts to achieve carbon neutrality by the 2050s.
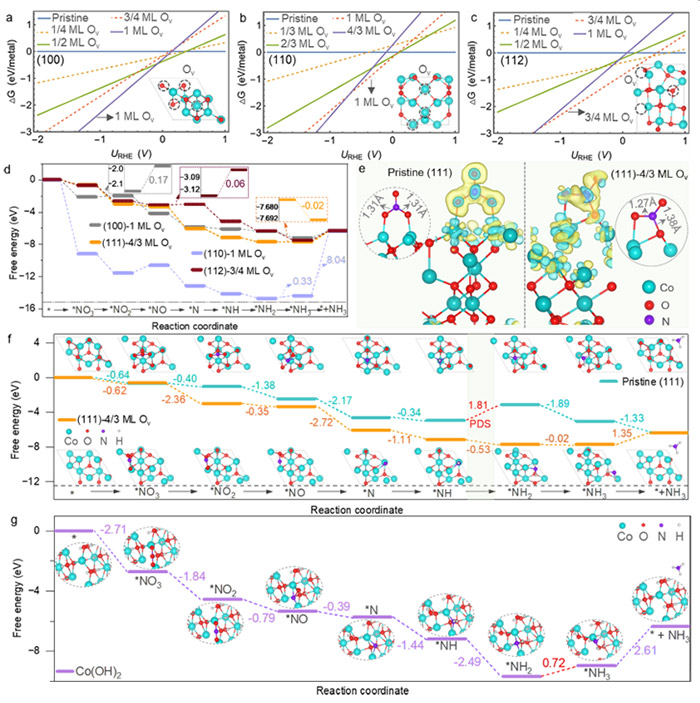
Theoretical calculations: 1D surface Pourbaix diagrams for Co3O4 (100), (110), and (112) surfaces, and reaction free energy diagrams for various intermediates on Co3O4 and Co(OH)2 surfaces during eNO3RR. Insets show charge density differences and N=O bond lengths. ©Hao Li et al.
| Title: | Facet-Dependent Evolution of Active Components on Spinel Co3O4 for Electrochemical Ammonia Synthesis |
|---|---|
| Authors: | Anquan Zhu, Heng Liu, Shuyu Bu, Kai Liu, Chuhao Luan, Dewu Lin, Guoqiang Gan, Yin Zhou, Tian Zhang, Kunlun Liu, Guo Hong, Hao Li, and Wenjun Zhang |
| Journal: | ACS Nano |
| DOI: | 10.1021/acsnano.4c06637 |
Hao Li (Profile of Dr. Li)
Advanced Institute for Materials Research (WPI-AIMR), Tohoku University
| E-mail: | li.hao.b8@tohoku.ac.jp |
|---|---|
| Webstie: | Hao Li Laboratory |