

07/31/2017
© 2017 Shigeyuki Takagi
A quartet of chemical compounds, each containing metal atoms surrounded by a remarkable nine hydrogen atoms, has been created by researchers at the AIMR1. These compounds are potentially useful for hydrogen-storage applications or as battery components, and one of them may also exhibit superconductivity.
Such nine-fold coordination of hydrogen is extremely rare. Until now, only two metals, rhenium and technetium, were known to form such complexes, with other metals coordinating fewer hydrogen atoms.
Shigeyuki Takagi, a member of Shin-ichi Orimo’s laboratory in the AIMR at Tohoku University, and colleagues used thermodynamic and electron distribution calculations to predict that the metals molybdenum, tungsten, niobium and tantalum should be able to coordinate nine hydrogen atoms.
The team then made the complexes by mixing the powdered metals with lithium hydride and forming the ingredients into pellets. They squeezed the pellets under a very high pressure with hydrogen gas and heated them to around 700 degrees Celsius for up to 2 days. After isolating the products, the researchers characterized them using techniques such as neutron diffraction and Raman spectroscopy.
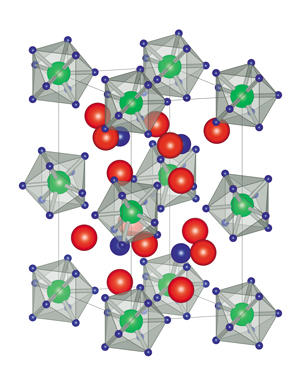
This analysis revealed that each metal atom was surrounded by nine hydrogen atoms, forming a shape known as a tricapped trigonal prism, which matched the team’s predictions (see image). These metal hydride clusters formed a regular crystalline lattice, with lithium and hydrogen atoms filling the gaps between them. This arrangement gives the compounds a very high hydrogen density, which makes them promising for storing hydrogen. “One of our next targets is to experimentally demonstrate the superior hydrogen-storage properties of our materials,” says Takagi.
The materials are all electrical insulators, but the team calculates that under even higher pressures the molybdenum complex could become metallic, allowing it to conduct a current. Moreover, the electrons mobilized under these conditions may even enable the material to superconduct at relatively high temperatures, says Takagi.
Calculations also indicated that the metal hydride complex in the molybdenum compound should be able to rotate, suggesting that lithium ions between the complexes might be able to move around inside the crystal and conduct electricity. “We recently conducted first-principles molecular dynamics calculations to examine lithium-ion conduction in the material and found a very high conductivity that exceeds those of currently known lithium-ion conductors,” says Takagi.
The team now hopes to experimentally confirm that this lithium-ion conduction occurs. “Subsequently, we will try to assemble all-solid-state lithium-ion batteries using our hydrides as solid-state electrolytes,” says Takagi. “We will also continue to further explore hydrogen-rich materials.”
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.