

06/27/2011
© 2011 ACS
Out of the array of rare earths and precious metals that fill automobile catalytic converters, a compound known as cerium oxide (CeO2) plays a special role. The amount of oxygen inside an engine’s combustion chamber determines the amount and composition of emissions that result from burning gasoline. Because CeO2 can both take in and release oxygen atoms without decomposing, this material is critical in balancing oxygen within the catalytic converter to bring emissions to their lowest levels possible. The emission-cleaning capacity of the bulky CeO2 crystals currently used in catalytic converters, however, is held back by the limited amount of oxygen that can be stored on their surfaces. Now, using organic molecules to precisely control CeO2 crystal growth, Tadafumi Adschiri from the WPI-AIMR and co-workers have prepared CeO2 ‘nanocubes’ that display almost triple the oxygen storage capacity of typical CeO2 crystals1.
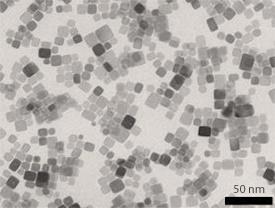
To produce these unique box-shaped crystals, the team turned to a technique known as supercritical hydrothermal synthesis. First, a CeO2 precursor, a short hydrocarbon chain and a water solvent were locked together in a pressure-resistant chamber and then heated to 400 °C. At supercritical temperature and pressure conditions, the organic molecules mix homogenously with the metal centers in aqueous solution, and attach to the most reactive faces of small CeO2 crystals, hindering growth in that direction. Transmission electron microscopy showed that this method generated distinct CeO2 cubes less than ten nanometers across (see image).
Testing the oxygen storage capacity of the ultrasmall cubes revealed surprising temperature-dependent properties. Whereas conventional irregular-shaped CeO2 crystals only take in oxygen when heated to 400 °C, Adschiri’s nanocubes adsorbed significant amounts of oxygen at a much lower 150 °C, indicating a much higher catalytic activity.
“Oxygen storage capacity is directly related to the mobility of oxygen in a material,” says Adschiri. Normally, metal oxides need high temperatures to make oxygen atoms travel through their crystal framework. But because the CeO2 nanocubes exhibit high mobility at lower temperatures, these compounds could potentially clean up emissions over a wider range of operating conditions. This could be a boon for vehicles and reduce the amount of precious metals used for catalytic emission cleaning.
Adschiri notes that controlling the growth of crystals by manipulating their exposed surfaces is the key to tuning the catalytic activity of CeO2. The researchers are now exploring how other capping molecules and different supercritical reactions conditions can lead to completely new methodologies for catalyst fabrication.
Zhang, J., Kumagai, H., Yamamura, K., Ohara, S., Takami, S., Morikawa, A., Shinjoh, H., Kaneko, K., Adschiri, T. & Suda, A. Extra-low-temperature oxygen storage capacity of CeO2 nanocrystals with cubic facets. Nano Letters 11, 361–364 (2011). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.