

10/26/2009
Solid metals usually exist as well-ordered crystals, but in recent years researchers have also produced stiff, strong, disordered metal alloys known as bulk metallic glasses (BMGs). However, it remains unclear exactly why certain combinations of metals form BMGs more readily than others.
Now, Takeshi Fujita, Mingwei Chen and co-workers at Tohoku University's Advanced Institute for Materials Research (AIMR) and the Miyagi National College of Technology in Natori1 have observed that when more than two types of metals are combined, their atoms may arrange in different types of clusters that aid the formation of a BMG. “Bulk metallic glass is a new type of high-strength material for engineering,” explains Chen. “It is also very easy to fabricate into various nanostructures in a supercooled liquid state.”
The team’s latest work was prompted by experiments showing that a two-metal alloy is more likely to form a BMG structure when small amounts of a third metal are added. However, it had so far remained difficult to scrutinize the structure of a multi-component BMG.
Fujita, Chen and their colleagues were able to examine the atomic structure of copper–zirconium (Cu–Zr) alloy samples containing varying amounts of added silver through the use of extended X-ray absorption fine structure (EXAFS) spectroscopy at the incredibly powerful SPring-8 synchrotron radiation facility in Hyogo, Japan.
“Without the advanced synchrotron source, we could not have detected the atomic structure of these disordered solids,” says Chen. “Our colleagues in Natori helped us to interpret the EXAFS spectra. I think multidisciplinary collaborations are very important in today’s cutting-edge research.”
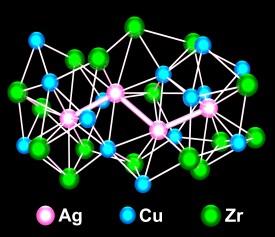
By combining their X-ray spectra with computer simulations, the researchers concluded that the atoms in their copper–zirconium–silver (Cu–Zr–Ag) BMG arranged themselves into two main types of structure: clusters with silver atoms at the center surrounded by a shell rich in zirconium, and clusters rich in copper throughout (Fig. 1). The atomic-scale heterogeneity favors glass formation.
These two types of clusters were seen in both the supercooled liquid and the solid glass state. Moreover, the fact that the clusters are close to the ideal sizes for efficient packing means that they represent an important step in glass formation. Different to conventional atomic clusters, the clusters in metallic glasses are not individually isolated. Instead, they always share ‘overlap’ atoms with neighboring clusters to form a dense and connected packing.
The findings highlight the importance of structural heterogeneity in forming amorphous solids. More generally, the results challenge the traditional notion that an atom in a solid usually prefers to be surrounded by atoms of different types.
Fujita, T., Konno, K., Zhang, W., Kumar, V., Matsuura, M., Inoue, A., Sakurai, T. & Chen, M.W. Atomic-scale heterogeneity of a multicomponent bulk metallic glass with excellent glass forming ability. Physical Review Letters 103, 075502 (2009). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.