

08/31/2009
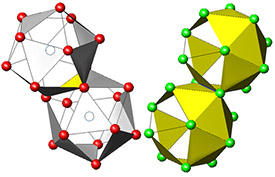
Fig. 1: Schematic diagram showing how the atoms in Zr–Cu metallic glass form icosahedral clusters that pack tightly together by sharing faces (left) or edges (right).
Metallic glasses, made by carefully cooling hot metal alloys so that they solidify without crystallizing, promise to revolutionize the materials industry. Thanks to an irregular, tightly packed internal arrangement of atoms, metallic glasses are moldable like plastic when heated, yet stronger than typical crystalline alloys.
Now, a team of international researchers from the Advanced Institute for Materials Research (AIMR) at Tohoku University in Japan and the Institut Polytechnique de Grenoble in France1 has used high-energy light to probe the internal structure of a metallic glass of zirconium (Zr) and copper (Cu). The results provide a guide for producing thicker alloy glasses with enhanced functionality.
The researchers, led by Alain Yavari, used a high-energy synchrotron X-ray source to ricochet photons off atoms buried deep inside a sample of Zr–Cu metallic glass. Mathematical interpretation of the resulting X-ray diffraction signals gave a description of how pairs of Zr–Zr, Cu–Cu and Zr–Cu atoms were distributed within the glass. By taking measurements of alloys with various compositions, the scientists were able to correlate changes in glass stability with changes in the atomic structure.
According to Yavari, the Zr–Cu metallic glass is an ‘ideal solid solution’ — that is, because the interactions among atoms are weak, the atoms can distribute randomly within the glass.
The random distribution of atoms creates a densely packed internal structure, but it also reduces the alloy’s resistance to crystallization — only very thin glasses can be cast from Zr–Cu mixtures. Recently, however, scientists found that adding a third component, aluminum (Al), to the hot alloy allowed stronger glasses to be cast at thicknesses of up to several millimeters.
In contrast to the ideal solution character of Zr–Cu, the researchers found that Zr–Cu–Al metallic glasses deviated markedly from ideal behavior. They observed that the Zr–Cu–Al glasses were comprised of finite regions of atomic order, providing the extra support needed to form thicker glasses. These ordered regions are believed to form due to attraction between the outer electrons of aluminum atoms and the larger zirconium atoms. The atoms arrange in shells resembling icosahedra, which pack together tightly in the glass (Fig. 1). Yavari and colleagues have previously observed the formation of such clusters in other Zr–Cu alloys.
The use of synchrotron light, Yavari says, was highly advantageous for analyzing the Zr–Cu–Al metallic glasses. Whereas conventional X-ray beams are completely absorbed at the surface of the material, light produced by a synchrotron has sufficient energy and intensity to penetrate into even thick samples, providing a deeper understanding of the unique internal structure of metallic glasses.
Georgarakis, K., Yavari, A.R., Louzguine-Luzgin, D.V., Antonowicz, J., Stoica, M., Li, Y., Satta, M., LeMoulec, A., Vaughan, G. & Inoue, A. Atomic structure of Zr–Cu glassy alloys and detection of deviations from ideal solution behavior with Al addition by X-ray diffraction using synchrotron light in transmission. Applied Physics Letters 94, 191912 (2009). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.