

When the current method for producing something is estimated to consume a staggering 1-2% of the annual global energy supply, it means we need to make a change. The Haber-Bosch process produces ample amounts of ammonia (NH3) - a valuable chemical compound that has a wide array of uses in fields such as agriculture, technology, and pharmaceuticals – while consuming a lot of energy.
A research team at Tohoku University has made a significant contribution to an alternate method for converting harmful nitrate pollutants in water into ammonia, addressing both environmental and energy challenges. By utilizing NiCuFe-layered double hydroxide (LDH) catalysts, the study provides an efficient method for cleaning contaminated water. This means cleaner water, reduced pollution, and more sustainable fertilizer and energy resources, which are directly beneficial to public health, food security, and climate protection.
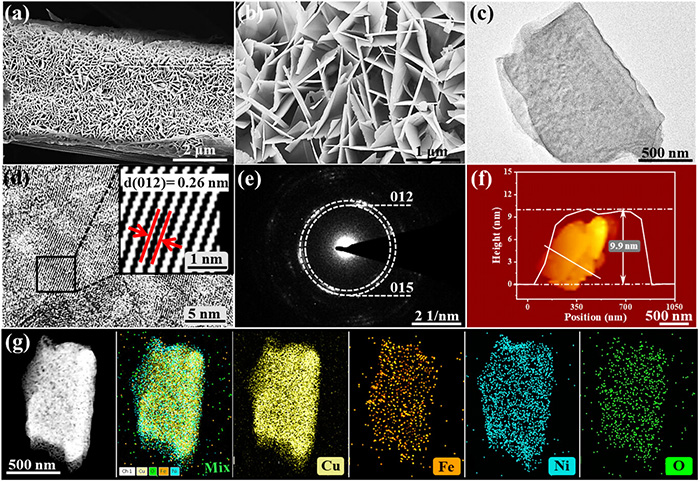
Structural characterization of NiCuFe-LDHs catalyst. a, b) SEM images, c) TEM image, d) HRTEM image, e) SAED pattern, f) AFM profiles, g) HAADF-STEM image, and the corresponding EDS element mapping. The inset in (d) shows the interplanar distance quantified from the HRTEM image. ©Yuan Wang et al.
The Haber-Bosch process currently produces almost all of the industrially produced ammonia in the world, but it has major downsides. Not only does it consume an exorbitant amount of energy, the process also releases carbon dioxide emissions as a byproduct, making it even more taxing on the environment. The electrocatalytic nitrate (NO3−) reduction reaction (NitRR) is an alternative option to produce ammonia that has existed for a while, but it never caught on due to being slow and inefficient. However, researchers at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) found a method to overcome this.
“We created NiCuFe-LDH nanosheets with Ni and Cu sites to help with electroreduction,” explains Professor Hao Li (WPI-AIMR). “The NitRR went from being too inefficient to even consider, to a Faradaic efficiency of 94.8%.”
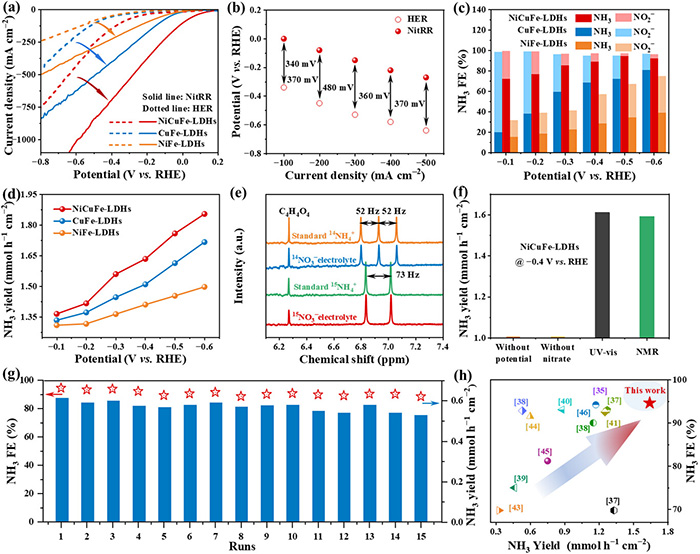
Electrochemical performance analysis of NiCuFe-LDHs nanosheets. a) LSV curves without (HER) and within (NitRR) 0.1 m KNO3 in 1.0 m KOH electrolyte. b) NitRR and HER overpotentials of NiCuFe-LDHs nanosheets at different current densities. The FE (c) and product yield (d) of CuFe-LDHs, NiFe-LDHs, and NiCuFe-LDHs nanosheets for NH3 synthesis in 1.0 m KOH with 0.1 m KNO3 electrolyte at different potentials. e) 1H NMR spectra using 15NO3− and 14NO3− electrolytes over NiCuFe-LDHs nanosheets. f) NH3 yield measured by NMR and UV–vis methods. g) The NH3 FE and NH3 yield in the durability test. h) The comparison of the NitRR performance of NiCuFe-LDHs nanosheets with previously reported advanced catalysts. ©Yuan Wang et al.
They used computational and theoretical analyses to explain the mechanism for this reaction, which involves the added Cu and Ni sites as the stars of the show. Furthermore, they tested a Zn-NO3− battery utilizing NiCuFe-LDH nanosheets to demonstrate its actual efficacy. It performed very well, with a Faradaic efficiency of 85.8%, a high yield of ammonia, and a power density so remarkable that it outperformed most previous reports (12.4 mW cm−2).
The next steps for this project will focus on scaling up and deepening mechanistic understanding. On the practical side, the catalyst performance needs to be validated in real nitrate-contaminated water systems and under continuous-flow reactor conditions to demonstrate industrial feasibility.
The findings were published in Advanced Functional Materials on September 4, 2025.
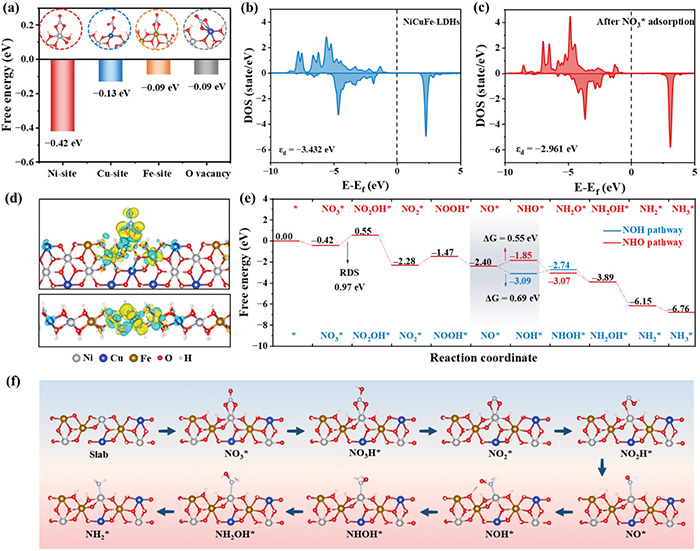
Theoretical insights into NitRR over NiCuFe-LDHs. a) Adsorption energy analysis of Ni, Cu, Fe, and O vacancy sites in NiCuFe-LDHs after NO3* adsorption. The DOS of the d-orbital of the Ni site in (b) NiCuFe-LDHs and c) NiCuFe-LDHs with NO3* adsorbate. Fermi energy was shifted to 0 eV. d) Charge density difference analysis of NiCuFe-LDHs with NO3* adsorbate in top and side view. e) Energy diagram of NitRR through NOH and NHO pathway over NiCuFe-LDHs. f) The adsorption configurations of the key intermediates during NitRR through the NOH pathway. ©Yuan Wang et al.
| Title: | Modulating Surface-Active Hydrogen for Facilitating Nitrate-to-Ammonia Electroreduction on Layered Double Hydroxides Nanosheets |
|---|---|
| Authors: | Bin Liu, Yuan Wang, Huiming Wen, Yuchen Wang, Heng Liu, Bo Da, Ke Li, Hao Luo, Hao Li and Kai Yan |
| Journal: | Advanced Functional Materials |
| DOI: | 10.1002/adfm.202519238 |
Hao Li (Profile of Dr. Li)
Advanced Institute for Materials Research (WPI-AIMR), Tohoku University
| E-mail: | li.hao.b8@tohoku.ac.jp |
|---|---|
| Webstie: | Hao Li Laboratory |