

Researchers at Tohoku University, the University of Barcelona, Tohoku Fukushi University, Waseda University, and Yamagata University have teamed up to develop a neuroengineering tool for designing neuronal circuits in vitro that mimic the integration-segregation capacity of the brain (Figure 1). The new technology paves the way to uncover the secrets of how the opposing dynamics coexist in a single circuit.
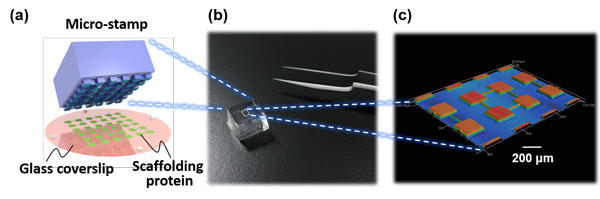
Fig. 1 (a) A schematic of microcontact printing used to pattern scaffolding proteins on a glass coverslip. Provided by Mr. Kei Wakimura. (b) A photograph of the micro-stamp. (c) Surface geometry of the micro-stamp. Copyright: Hideki Yamamoto
What makes the brain unique is its capacity to switch between different states. This happens internally and in response to external stimuli. For instance, stimuli coming from sight, sound or smell are processed in a segregated manner in the cerebral cortex, with the outcomes later integrated totally or partially according to the information the brain needs.
We integrate images and sounds while watching a movie, discarding smells and other stimuli. But when we notice the smell of burning, the brain activates alert mechanisms to integrate and analyze all available information and act on this.
Despite the importance of balancing the integrated and segregated states, however, the biophysical mechanisms of how this is realized in a single circuit are not well understood. Additionally, the sensitivity of the integration-segregation balance to the number of physical connections between brain regions is still unknown.
The in vitro brain model that the researchers developed consists of four interconnected modules. Each module represents a specialized neuronal circuit, such as vision or hearing. These four modules are covered by adhesive proteins where neurons develop. These neurons are connected among them inside a module and with other neurons in separate modules.
Precision neuroengineering allows controlling the connections that go from one module to another, enabling a gradual control of the physical coupling between modules (Figure 2). The stimuli in this model correspond to neural spontaneous activations.
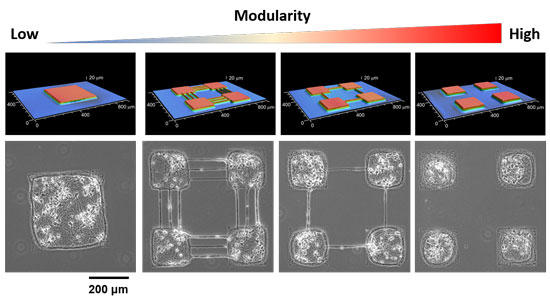
Fig. 2 Controlling the modularity of living neuronal networks (bottom) using different micro-stamps (top). Copyright: AAAS and Hideki Yamamoto.
“This study shows the potential of in vitro tools and biophysical models to progress in the understanding of collective phenomena in such a fascinating and rich complex system like the brain” says Jordi Soriano, a researcher at the University of Barcelona.
Using fluorescence calcium imaging to detect neuronal activations, the researchers studied the capacity of the circuit to spontaneously realize integration or segregation for different connectivity degrees among modules and other variables (Figure 3). They saw that the circuit is fully integrated or segregated when the number of connections between modules is too big or too small.
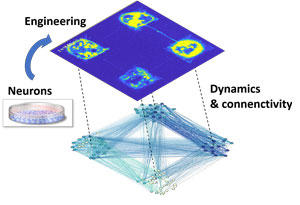
Fig. 3 Analysis of neural dynamics by fluorescent calcium imaging (top) and functional connectivity estimated from the network dynamics (bottom). Copyright: Jordi Soriano
The optimal circuit that balances the two states appeared when the four modules had a connectivity just below the minimum to integrate, so that neural activity pulses could be reinforced in each modules to complete integration.
“Effectually, this optimal circuit –spontaneously activated–- works in a regime where integration and segregation coexist. Of course, the observed dynamics is still far from the complexity in the actual brain, but we were able to gain insight into the fundamental mechanisms shaping brain dynamics,” said Hideaki Yamamoto of Tohoku University.
Title: Impact of modular organization on dynamical richness in cortical networks
Authors: H. Yamamoto, S. Moriya, K. Ide, T. Hayakawa, H. Akima, S. Sato, S. Kubota, T. Tanii, M. Niwano, S. Teller, J. Soriano, A. Hirano-Iwata
Journal: Science Advances
DOI: 10.1126/sciadv.aau4914
Hideaki Yamamoto
Advanced Institute for Materials Research, Tohoku University
| E-mail : | hideaki.yamamoto.e3@tohoku.ac.jp |
|---|---|
| Website : |