

A new catalyst structure offers a potential pathway toward more cost-effective hydrogen production via water electrolysis. The material centers on mesoporous single-crystalline Co3O4 doped with atomically dispersed iridium (Ir), designed for the acidic oxygen evolution reaction (OER).
Iridium is known for its OER performance but is both scarce and expensive. Efficient use of Ir while maintaining stability is a major challenge for scaling up electrolyzer technologies. This study proposes a solution through a material that maximizes atomic-level efficiency.
The catalyst features a mesoporous spinel structure that allows for high Ir loading (13.8 wt%) without forming large Ir clusters. This configuration enables the formation of Co–Ir bridge sites, which show high intrinsic activity under acidic OER conditions.
Computational analysis indicates that under reaction conditions, oxygen intermediates (O*) fully cover Co3O4 surfaces, which usually passivates Co sites. However, Ir doping reactivates these sites, while simultaneously enhancing the structural integrity of the catalyst.
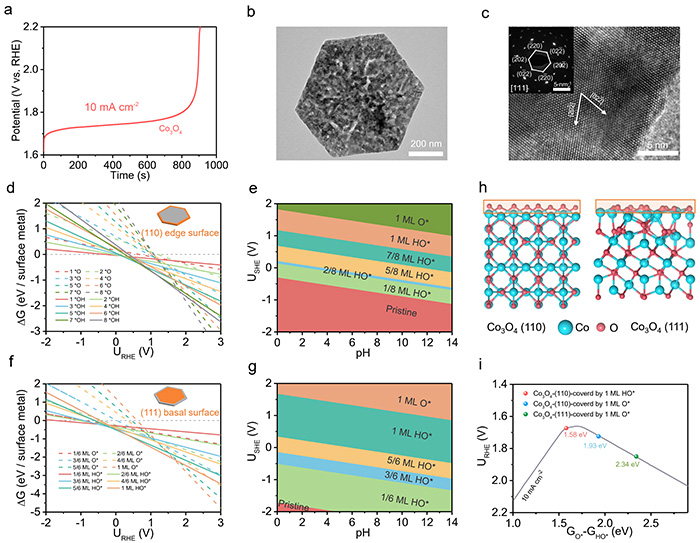
Deactivation of Co3O4 acidic OER catalyst. (a) Chronopotentiometry curve of Co3O4 at a current density of 10 mA cm–2 tested on a glassy carbon electrode. (b) TEM and (c) HRTEM images of Co3O4 after chronopotentiometry testing, with the inset showing the SAED pattern. Calculated 1D surface Pourbaix diagrams for the (110) edge surface (d) and (111) basal surface (f) of Co3O4 as a function of potential (pH = 0; T = 298.15 K). Calculated 2D surface Pourbaix diagrams for the (110) edge surface (e) and (111) basal surface (g) of Co3O4 as a function of potential (vs RHE) and pH (T = 298.15 K). (h) Structural models of the Co3O4(110) edge surface and (111) basal surface covered by 1 ML O* (side view). (i) Location of the Co3O4(110) edge surface and (111) basal surface with different surface coverages on the volcano plot. All relevant computational structures are available in the Digital Catalysis Platform (DigCat) database . ©Yong Wang et al.
. ©Yong Wang et al.
Leaching of both Ir and Co during reaction was significantly reduced. Compared to conventional Ir/Co3O4 catalysts, Ir and Co loss was lowered to approximately one-fourth and one-fifth, respectively. The catalyst also maintained performance for over 100 hours with an overpotential (η10) of just 248 mV.
“The mesoporous architecture plays a crucial role,” explains Professor Hao Li, who led the study. “It provides space for single-atom Ir loading and helps create a stable environment for catalytic activity.”
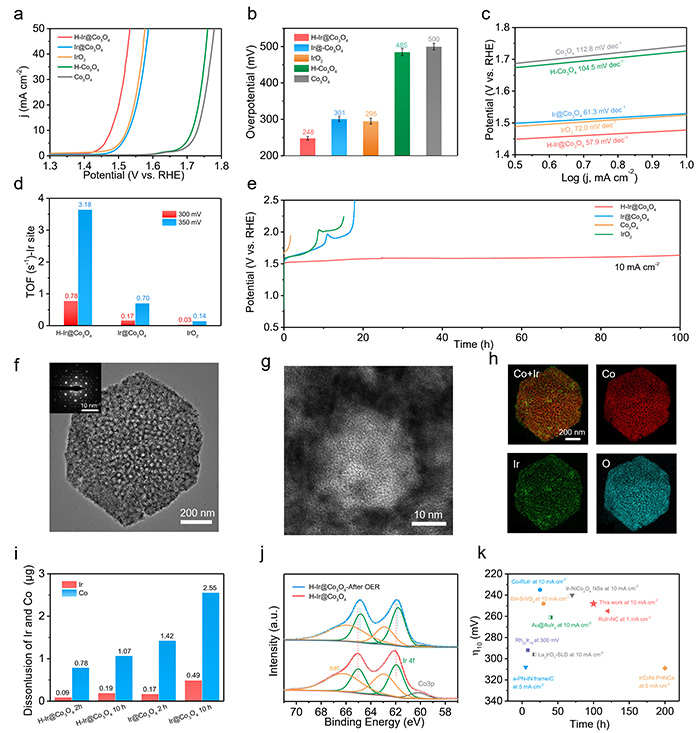
Electrocatalysis OER of H–Ir@Co3O4in 0.5 M H2SO4. (a) iR-corrected polarization curves, (b) overpotentials required for j = 10 mA cm–2 and (c) the corresponding Tafel plots of the catalysts. (d) TOFs based on Ir calculated at overpotentials of 300 or 350 mV. (e) Chronopotentiometry curves of H–Ir@Co3O4, Ir@Co3O4, H–Co3O4, and IrO2 at a current density of 10 mA cm–2. (f, g) bright-field TEM images (the inset is the SAED spots) and (h) EDS mapping of H–Ir@Co3O4 after 100 h of OER testing. (i) Leaching mass of Ir and Co from H–Ir@Co3O4 and Ir@Co3O4 after 2 and 10 h of OER at a current density of 10 mA cm–2 (the catalysts were loaded on a glassy carbon electrode). (j) Ir 4f XPS spectra of H-IrO2@Co3O4 before and after OER. (k) η10 and stability comparisons of H–Ir@Co3O4 and state-of-the-art OER catalysts in acid electrolyte. ©Yong Wang et al.
The research combines experimental data with computational modeling, and key findings are available through the Digital Catalysis Platform , a resource developed by the Hao Li Lab to support catalyst discovery.
, a resource developed by the Hao Li Lab to support catalyst discovery.
This work was supported by the Tohoku University Support Program. Future research will focus on tuning the doping level, scaling up the synthesis process, and exploring integration into commercial electrolyzer systems.
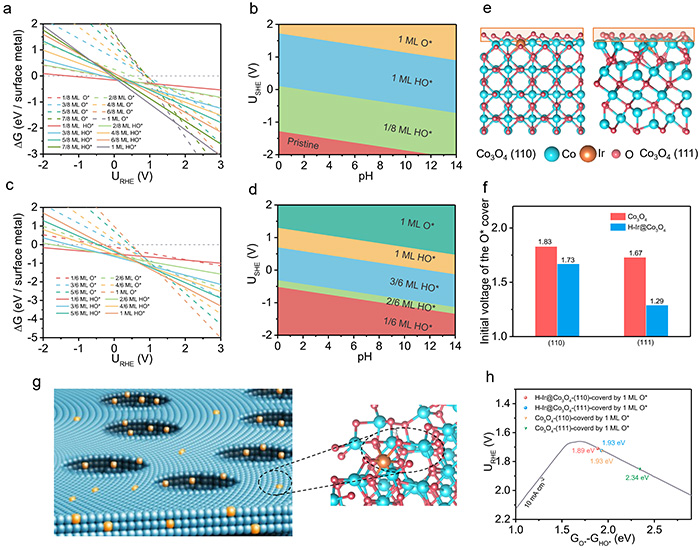
DFT calculations of H–Ir@Co3O4. Calculated 1D surface Pourbaix diagrams for the H–Ir@Co3O4(110) surface (a) and (110) surface (c) as a function of potential (pH = 0; T = 298.15 K). Calculated 2D surface Pourbaix diagrams for the (111) surface (b) and (111) surface (d) of H–Ir@Co3O4 as a function of potential and pH (T = 298.15 K). (e) Structural models (side view) of the H–Ir@Co3O4(111) and (110) surfaces covered by 1 ML O*. (f) Formation voltages of Co3O4 and H–Ir@Co3O4(111) and (110) surfaces covered by 1 ML O*. (g) Schematic of the structure and active site of H–Ir@Co3O4. (h) Location of Co3O4 and H–Ir@Co3O4(111) and (110) surfaces covered by 1 ML O* in the volcano model. Computational structures are also available in the Digital Catalysis Platform (DigCat) . ©Yong Wang et al.
. ©Yong Wang et al.
| Title: | Mesoporous single-crystalline particles as robust and efficient acidic oxygen evolution catalyst |
|---|---|
| Authors: | Yong Wang, Yunpu Qin, Sijia Liu, Yongzhi Zhao, Luan Liu, Di Zhang, Shangqing Zhao, Jianfang Liu, Jie Wang, Yadong Liu, Haoyang Wu, Baorui Jia, Xuanhui Qu, Hao Li, Mingli Qin |
| Journal: | Journal of the American Chemical Society |
| DOI: | 10.1021/jacs.4c18390 |
Hao Li (Profile of Dr. Li)
Advanced Institute for Materials Research (WPI-AIMR), Tohoku University
| E-mail: | li.hao.b8@tohoku.ac.jp |
|---|---|
| Webstie: | Hao Li Laboratory |