

An international group of researchers has developed a novel approach that enhances the efficiency of the oxygen evolution reaction (OER), a key process in renewable energy technologies. By introducing rare earth single atoms into manganese oxide (MnO2), the group successfully modulated oxygen electronic states, leading to unprecedented improvements in OER performance.
Their findings were published in the journal Nano Energy on June 10, 2024.
Transition-metal-based oxides have been widely explored for their potential as active OER catalysts. However, the capacity of these catalysts is hindered by the adsorbate evolution mechanism, which limits the effective release of oxygen (O2) during the reaction.
“We constructed localized asymmetric gadolinium-oxygen-manganese units on MnO2, which helps accumulate electrons at oxygen sites,” notes Hao Li, corresponding author of the paper and an associate professor at the Advanced Institute for Materials Research (WPI-AIMR) at Tohoku University. “By doing this, the catalysts achieve a lower overpotential and maintain stability over time, making it a suitable alternative to traditional catalysts such as ruthenium dioxide (RuO2).”
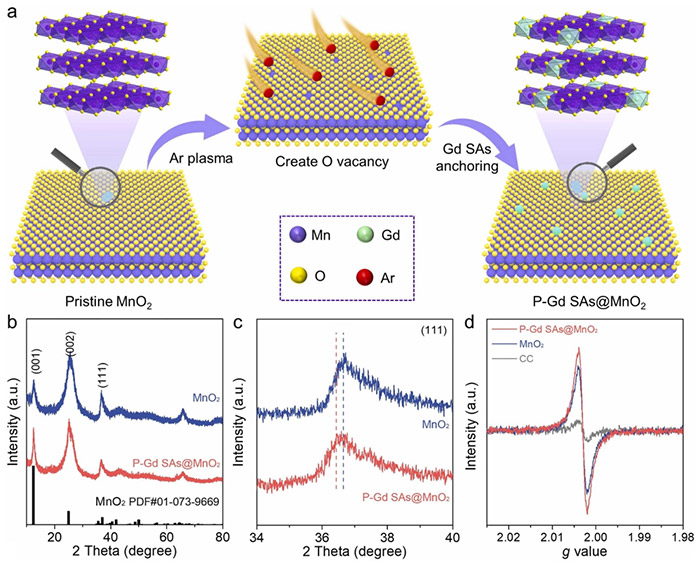
(a) The schematic route for the synthesis of P-Gd SAs@MnO2 nanosheets; (b, c) XRD patterns of P-Gd SAs@MnO2 and MnO2; (d) EPR spectrum of P-Gd SAs@MnO2, MnO2, and blank CC. ©Hao Li et al.
Hao Li and his colleagues employed an argon plasma-assisted strategy to introduce rare earth elements on the catalyst surface. In this strategy, argon gas is ionized, energizing and helping break the argon atoms into ions and electrons, thereby making it easier to interact with and modify materials.
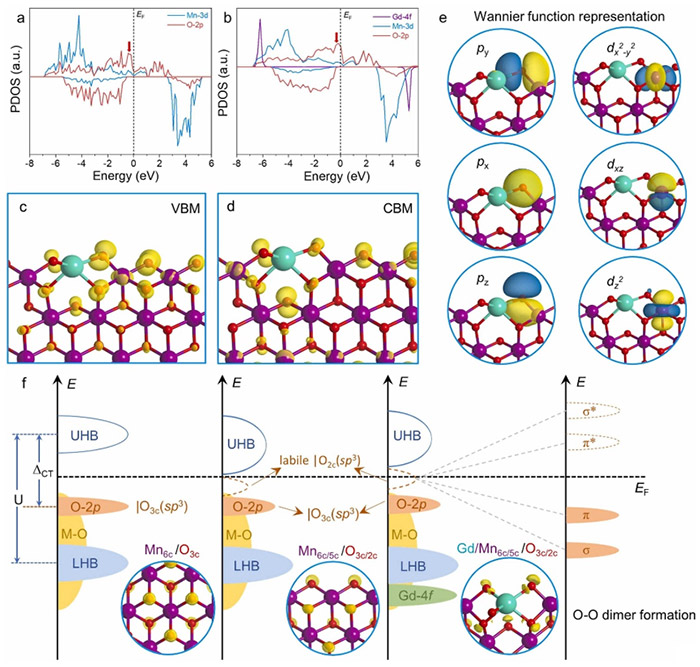
PDOS diagrams of (a) MnO2 slab model and (b) Gd-MnO2 slab model with [Gd−O−Mn] unit site. (c)-(d) VBM and CBM diagrams of Gd-MnO2 with Kohn-Sham orbital population (iso-surface 0.002 e/Å3). (e) Projected MLWF plots of O2c and Mn5c sites in [Gd−O−Mn] unit (iso-surface 1.5 e/Å3). (f) Mechanism of (O-O) dimer formation for OER catalysis, showing ELF plots for bulk MnO2, slab MnO2 model, and Gd-MnO2 slab model (iso-surface 0.7 e/Å3). Pink, green, red, and white spheres denote Mn, Gd, O, and H, respectively. ©Hao Li et al.
“We have addressed the challenges associated with the adsorbate evolution mechanism that limits the performance of transition-metal-based oxides like MnO2,” adds Di Zhang, co-author of the study and a Specially Appointed Assistant Professor at WPI-AIMR. “By improving the understanding of the structure-activity relationship under the lattice oxygen mechanism, the research provides a foundation for more effective catalyst design.”
Building on the success of this study, the group plans to extend their methodology to a variety of electrochemical reactions. This approach will help further decipher unique structure-activity correlations, ultimately contributing to the design of even more effective and high-performance electrocatalysts.
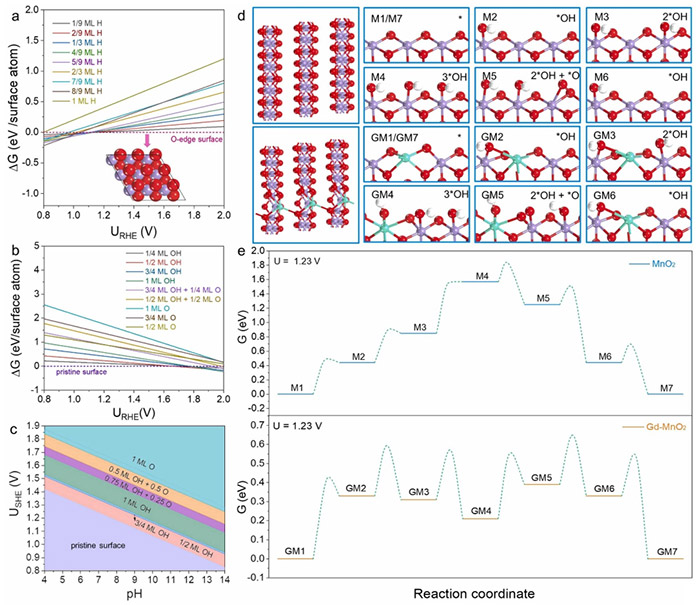
Surface Pourbaix diagrams: (a) H coverage for MnO2 slice with Mn6c-O3c chain, (b) oxygen species coverage for MnO2 slab with Mn5c and O2c sites. (c) 2D surface Pourbaix diagram showing URHE vs. pH. (d) Geometric structures of MnO2 and Gd-MnO2 with various oxygen intermediates. (e) OER free energy diagrams for MnO2 and Gd-MnO2 at 1.23 V. Pink, green, red, and white spheres denote Mn, Gd, O, and H, respectively. ©Hao Li et al.
| Title: | Atomic rare earths activate direct O-O coupling in manganese oxide towards electrocatalytic oxygen evolution |
|---|---|
| Authors: | Meng Li, Xuan Wang, Di Zhang, Yujie Huang, Yijie Shen, Fei Pan, Jiaqi Lin, Wei Yan, Dongmei Sun, Kai Huang, Yawen Tang, Jong-min Lee, Hao Li, Gengtao Fu |
| Journal: | Nano Energy |
| DOI: | 10.1016/j.nanoen.2024.109868 |
Hao Li (Profile of Dr. Li)
Advanced Institute for Materials Research (WPI-AIMR), Tohoku University
| E-mail: | li.hao.b8@tohoku.ac.jp |
|---|---|
| Webstie: | Hao Li Laboratory |