Research | Adschiri Laboratory, Institute of Multidisciplinary Research for Advanced Materials, Tohoku University

Research
Supercritical Hydrothermal Metal Oxide Particle Synthesis
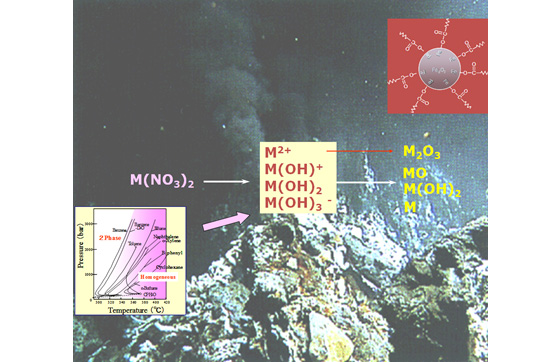
Water under the condition of temperature above 374℃ and pressure higher than 22.1 MPa is supercritical state.
Sea water near volcanos as shown in the photo is an example of water of the supercritical state.
Supercritical water has special features that normal water dose not have.
For example, the dielectric constant is as low as polar organic solvents.
Under such a condition, the equilibrium of metal aqueous solution shifts to oxide side.
Using this equilibrium shift, we can synthesize nanoparticles of metal oxides.
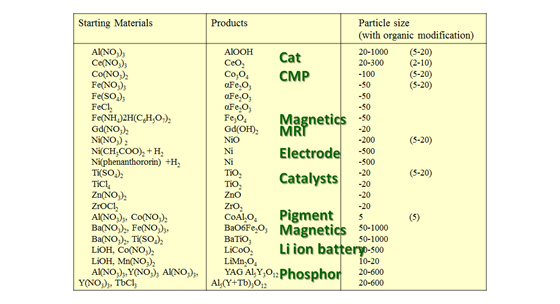
We have synthesized metal oxide nanoparticles (NPs) such as ceria (CeO2), titania (TiO2), magnetite (Fe3O4), barium titanate (BaTiO3).
These particles can be used for wide rage of materials, for instance, electrode materials, automobile, electronic component, catalyst, and magnetic materials.
Organic Surface Modification of Metal Oxide Particles
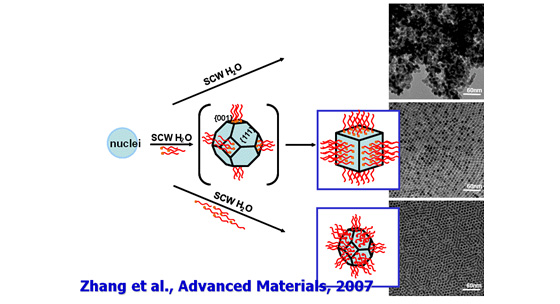
One of the most typical features of supercritical water is that water can mix with organic compounds. This feature enables the reaction using metal salt aqueous solution and organic compounds. We have succeeded the surface modification of metal oxide nanoparticles using carboxylic acids and amines.
The surface modification has been considered a difficult task to disperse the NPs in organic solvents or polymers, especially for the particles synthesized under hydrothermal conditions. This is because metal oxide particle surface is hydrophilic, and for the case of NPs, it shows extremely high surface energy, which leads to the formation of aggregates.
The supercritical hydrothermal synthesis overcomes this difficulty.
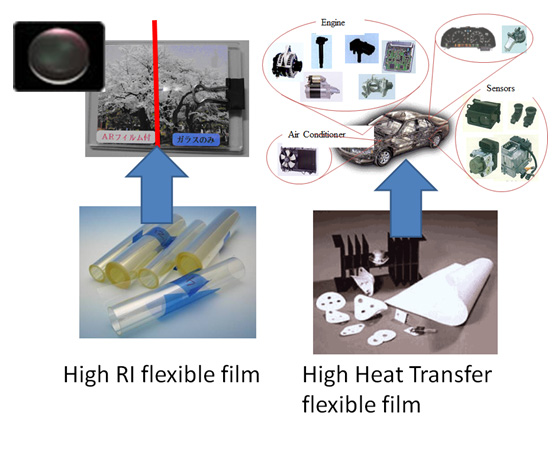
Through this modification, organic-inorganic hybrid materials can be developed. For instance, surface modified metal oxide nanoparticles are dispersed in polymers with high weight fraction.
A thin film with high thermal conductivity can be fabricated from this polymer-inorganic hybrid material.
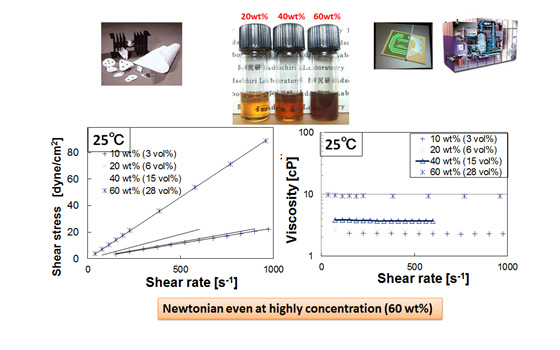
The high affinity of surface modified nanoparticles to organic solvents enables perfect dispersion in organic solvents with high weight fraction.
In addition, such surface modified nanoparticle solutions show lower viscosity compared with other inorganic nanoparticle solutions at the same weight fraction.
Such a low viscosity may be attributed to the surface character of modified nanoparticles. We are now trying to elucidate the mechanism of this phenomenon with the math- and interface-units in WPI-AIMR, Tohoku University.
Establishment of Science & Technology for Supercritical Hydrothermal Synthesis and Synthesized Materials
Our method to synthesize organic-inorganic hybrid materials is being applied to the development of materials by domestic and overseas companies.
For the acceleration of the development, basic principle and technology for hybrid materials are required.
For example, we're establishing the thermodynamics for nanoparticles.
For the observation of supercritical hydrothermal synthesis, we're developing a measurement system using neutron ray.
To enlarge the application of hybrid materials, we have flow reactor systems for increasing the throughput of the materials (above ton/year). We also study the design methodology of such systems.
Moreover, a technology to handle nanoparticles like atom and ion and to crystalize on demand (control of exposed surface and structure) is being developed.