

09/28/2020
Adapted, with permission, from Ref. 1. Copyright (2020) American Chemical Society.
Hybrid silicon anodes for lithium-ion batteries that overcome problems that had previously beset silicon anodes have been created by AIMR researchers1. This advance will help increase the energy storage of next-generation lithium-ion batteries.
Lithium-ion batteries are ubiquitous today, being used to power everything from smart phones to electric vehicles. However, the graphite-based anodes used in today’s batteries are approaching the limit of their development, and a new anode material is needed to realize lithium-ion batteries with even higher energy capacities.
Silicon is a promising alternative anode material because it is cheap and abundant and has a large reversible capacity and a good working potential. “Exploiting these advantages of silicon will result in a further breakthrough in the energy density of lithium-ion batteries,” predicts Mingwei Chen of the AIMR at Tohoku University.
But silicon suffers from three significant problems. One is that it expands and contracts a lot during charging and discharging, changing its volume by more than a factor of three. This degrades the electrodes and can result in loss of electrical contact inside the battery. Another problem with silicon is its low conductivity, which reduces the charging and discharging rate. A third problem is that the protective film that forms between silicon and the electrolyte during the first few charging cycles is unstable.
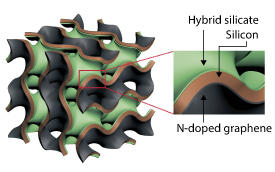
Now, the team led by Chen has overcome all these problems by designing and producing a nanoporous silicon anode that has a sandwich-like structure. In this anode, the silicon is coated on one surface by nitrogen-doped graphene and on the other surface by a hybrid silicate (see image).
Both surfaces play important roles in overcoming the above-mentioned limitations of silicon. “The nanoporous graphene in the anode acts as a flexible and conductive backbone that simultaneously accommodates silicon’s volume change and improves its electrical conductivity,” explains Chen. “On the other hand, the hybrid silicate coating acts as a protective outer shell, enhancing the robustness and suppleness of the electrode and facilitating the formation of stable films on the anode surface.”
In tests involving half and full cells, the anode exceeded the team’s expectations, realizing both an excellent cycling performance and rate capability. “We were really surprised by the ultrahigh stability of this hybrid silicon anode,” says Chen. “It was stable over 10,000 cycles and had a high capacity and ultrahigh charging rate.”
The team now plans to stack several free-standing graphene−silicon sheets to boost the energy storage capacity.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.