

08/31/2020
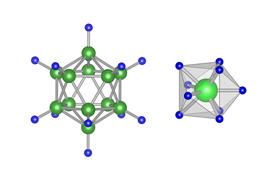
Reprinted from Ref. 1, with the permission of AIP Publishing.
Researchers at the AIMR have hit upon a way to get polyanions to rotate at much lower temperatures than normal, opening the possibility of realizing solid-state batteries with short charging times1.
Most commercial batteries use liquid electrolytes to convey charges between their electrodes, but if solid electrolytes could be used instead, batteries would be both safer and able to store more energy at the same volume.
Solid-state batteries require solid electrolytes that have high conductivities for ions. One promising class of material for realizing this is ionic conductors that contain polyanions — ions that have multiple negatively charged groups. The polyanions rotate at high temperatures, and this motion assists the conduction of positive ions through the material. But this rotation, and hence the high ion conductivity, does not occur at room temperature, making it impractical for many applications.
Now, four researchers at Tohoku University, including two from the AIMR, have realized a room-temperature lithium-ion conductivity in a hydride that is more than three times higher than the highest value obtained to date.
The results exceeded the team’s expectations. “We were surprised since the activation energy for rotation was lower than we had expected,” says Shigeyuki Takagi of the Institute for Materials Research at Tohoku University. “We anticipate that our finding will promote the development of rechargeable batteries with very short charging times.”
Previous studies had used bulky anions as the rotating elements, which require significant activation energies to get them to rotate. In contrast, Takagi and his team achieved the high lithium-ion conductivity at room temperature by using the transition metal molybdenum surrounded by nine hydrogen atoms. Because the rotating components are lightweight hydrogen atoms, the rotation occurs even at room temperature (see image).
The rotation of the hydrogen atoms is actually not a true rotation, but a pseudorotation involving just small displacements of the hydrogen atoms. This further promotes the lowering of the activation energy.
“The pseudorotation enhances the dynamic disorder of the hydrogen atoms, thereby increasing the entropy, which thermodynamically stabilizes the pseudorotating high-temperature phase at low temperature,” says Takagi.
The researchers anticipate that the same strategy should be applicable to other polyanionic materials. “Because the mechanism is quite general, we think that it should be useful for developing all-solid-state secondary batteries with different carriers, such as sodium and magnesium ions,” notes Takagi.
The team is now planning to experimentally demonstrate room-temperature superionic conduction, which has been predicted theoretically.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.