

12/25/2017
© 2017 Zhili Wang
An easy way to enhance the effectiveness of nanoporous gold catalysts that involves sculpting their surfaces by applying a cycling voltage to the catalyst has been demonstrated by AIMR researchers1.
Gold is renowned for its low chemical reactivity, which is why you need not fret that your gold ring will tarnish or corrode. But if gold is made small enough — on the order of nanometers — it begins to take part in chemical reactions and can be used as a catalyst to speed up reactions.
Gold nanoparticle catalysts have been widely investigated, but a more stable form of gold has recently been attracting attention — gold riddled with nanoscale wormholes, known as nanoporous gold. It is made by forming an alloy of gold and silver and then using a strong acid to strip away the silver, leaving the gold with pores that are tens of nanometers in diameter.
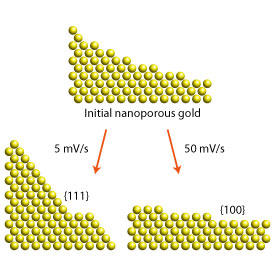
A team led by Mingwei Chen of the AIMR at Tohoku University has found a way to enhance the catalytic properties of nanoporous gold by tuning the surface structure of its pores. During fabrication, they applied a cycling voltage to nanoporous gold and were able to obtain different kinds of surfaces by varying the scan rate. For example, when the team varied the voltage up and down at a rate of 5 millivolts per second, they obtained {111} surfaces, the closest-packed planes of gold, but when they cycled it at ten times that rate, they obtained {100} surfaces (see image).
The team demonstrated their catalyst by using it to catalyze the ethanol oxidation reaction, an important reaction for realizing fuel cells based on the environmentally friendly fuel ethanol. Nanoporous gold containing an abundance of {111} surfaces had a higher activity than conventional nanoporous gold. Indeed, it had the highest activity for the reaction of any gold catalyst reported to date.
“This not only opens up a new avenue to improve the catalytic activity of three-dimensional nanoporous catalysts by surface engineering, but also provides a direction to develop three-dimensional nanoporous catalysts with controllable surface structures for chemical and electrochemical reactions related to energy and the environment,” says Zhili Wang, a postdoctoral fellow of the team.
Unlike previous techniques, the method does not use a surfactant, which is important because surfactants can be hard to flush out from the pores and residual surfactant can lower the catalytic activity of nanoporous catalysts.
The team will use the method to produce other nanoporous catalysts. “We intend to prepare three-dimensional nanoporous platinum and silver with controllable surface structures for carbon dioxide reduction and nitrogen fixation,” says Wang.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.