

02/27/2017
© 2017 Yoshikazu Ito
A carbon-based material has been transformed into a catalyst that uses electricity to split water, producing clean-burning hydrogen gas1.
“Electrolysis of water is becoming more important as a way to store energy from renewable sources such as wind, sunlight and water,” says Yoshikazu Ito of the AIMR at Tohoku University. “Since conventional platinum electrodes are prohibitively expensive, we are exploring the use of metal-free electrodes.”
The new catalytic electrode is based on graphene — atom-thin sheets of carbon atoms that are strong, flexible and electrically conductive. Theoretical predictions suggest that adding atoms of sulfur, phosphorus and nitrogen should make graphene-based electrodes as effective as platinum ones. But, in practice, the performance of these ‘doped’ graphene electrodes has not met these expectations.
Now, a team led by AIMR’s Ito and Mingwei Chen has improved these graphene electrodes by carefully controlling the amounts and positions of different dopant atoms in the material.
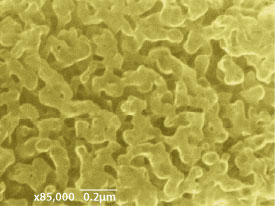
Using nickel nanoparticles as porous templates, the researchers added different mixtures of gases that contained carbon, hydrogen, sulfur, nitrogen or phosphorus. When heated to 750 degrees Celsius, this coated the nickel’s nanopores with a layer of graphene three to six atoms thick and included various amounts of dopant atoms. Dissolving the nickel template with acid left graphene with pores ranging in size between 50 and 2,000 nanometers. These pores provide a larger surface area for the hydrogen ions in water to access catalytically active chemical sites within the material.
Employing templates with smaller pores produced graphene that was more curved, creating defects in its structure that could be occupied by dopant atoms. Graphene with pores between 50 and 100 nanometers across had the highest loading of all three dopant atoms, in positions that were more chemically reactive (see image).
The team measured the hydrogen-generating performance of various nanoporous graphene samples, variously containing one, two or all three types of dopants, and compared them with undoped nanoporous graphene. They found that the tri-doped nanoporous graphene offered the greatest improvement in catalytic activity, although it did not match that of a platinum electrode.
The researchers suggest that the presence of three different types of dopant atoms alters the distribution of electrical charge on graphene’s surface, offering a balance of negative and positive regions that first adsorb hydrogen atoms (H) and then desorb hydrogen molecules (H2) during the reaction. Using pore size to control this doping should allow them to fine-tune the material and further boost its catalytic properties.
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.