

10/26/2015
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
A graphene electrode loaded with catalytic nanoparticles significantly improves the performance of rechargeable lithium–oxygen batteries, AIMR researchers have found.
Lithium–oxygen batteries offer ten times higher energy densities than conventional lithium batteries. They are also much lighter because one of their crucial components – oxygen – comes from the air around them, rather than being stored in the cathode.
When a lithium–oxygen battery discharges, its lithium metal anode produces a current of electrons by freeing positive lithium ions. These ions migrate through a liquid electrolyte and enter a porous cathode, where they react with oxygen to form lithium peroxide (Li2O2). This chemical reaction is reversed during charging.
But conventional porous carbon cathodes often break down during these reactions, generating other lithium compounds that reduce the battery’s efficiency. Cathodes made from one-atom-thick carbon flakes, known as graphene, offer greater stability and conductivity, but require high charging voltages of 4.5 volts or more. Catalysts such as ruthenium oxide (RuO2) nanoparticles can reduce this voltage by breaking down stored Li2O2, but the nanoparticles agglomerate into inactive clumps after a few charging cycles.
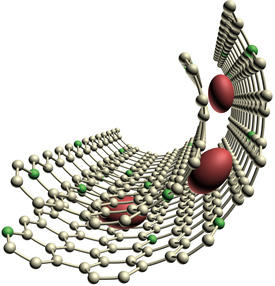
Now, Xianwei Guo, Mingwei Chen and colleagues from the AIMR at Tohoku University have dramatically improved the energy efficiency and lifetime of a lithium–oxygen battery by encapsulating RuO2 nanoparticles with graphene1.
The researchers created a porous nickel framework as a template for their cathode and then added the chemical pyridine at a temperature of 800 degrees Celsius. As the pyridine decomposed, it coated the insides of the template with a form of graphene that contained some nitrogen atoms. After loading this nitrogen-doped graphene with RuO2 nanoparticles roughly 3–5 nanometers in diameter, the researchers added a second layer of nitrogen-doped graphene. Etching away the nickel left a porous cathode that contained the RuO2 nanoparticles sandwiched between two graphene layers (see image). Lithium ions penetrated deep into these pores and combined with oxygen at nitrogen-binding sites.
A lithium–oxygen battery containing this cathode required an average charging voltage of just 3.7 volts, and sustained a high energy capacity for more than 100 charge–discharge cycles. The team confirmed that the RuO2 nanoparticles remained safely encapsulated between the graphene sheets and that very few unwanted lithium by-products formed.
The researchers hope to improve their cathode by using more economical materials. “We are trying to develop low-cost cathodes with cheap catalysts for higher efficiency, longer lifetimes and lower charging voltages to realize the commercialization of lithium–oxygen batteries,” says Guo.
Guo, X., Liu, P., Han, J., Ito, Y., Hirata, A., Fujita, T. & Chen, M. 3D nanoporous nitrogen-doped graphene with encapsulated RuO2 nanoparticles for Li–O2 batteries. Advanced Materials 27, 6137–6143 (2015). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.