

06/29/2015
© 2015 Serge Ostrovidov
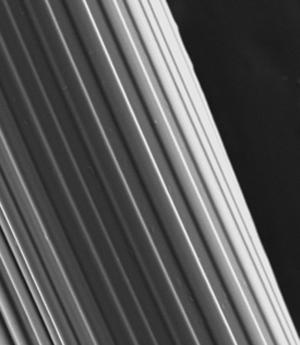
Using microfluidic spinning, AIMR researchers have grown hydrogel fibers that induce the hierarchical cellular organization commonly found in skeletal muscles and blood vessels1.
Tissue engineering is the cornerstone of regenerative medicine. Developing suitable scaffolds on which to grow anything from bone cells to heart cells is critical for growing artificial organs that the body will accept. But this is not easy. “Many tissues in the body vary greatly in composition, cell type and organization,” explains Serge Ostrovidov of the AIMR at Tohoku University. “Consequently, it is very challenging to build a material with varying properties able to support different cell types with their specific needs and organization.”
Recognizing that conventional methods for fabricating fibers were inadequate for making the grooved structures needed to support complex cellular environments, Ostrovidov and his AIMR colleagues together with overseas collaborators turned to microfluidic spinning.
Microfluidic spinning affords considerable control over the macro- and microscale characteristics of fibers and can produce fibers that are centimeters to meters in length and micrometers in diameter. Ostrovidov notes that the ease and versatility of the technique enabled his team to concentrate on research without worrying about lengthy setting-up and waiting times.
The choice of fiber material was critical: instead of using alginate, a common microfluidic spinning material that strongly repels cells, Ostrovidov and colleagues modified the natural hydrogel gelatin with methacrylic groups to create the polymer gelatin methacryloyl (GelMA).
To support complex cellular organizations, such as those in muscles or blood vessels, fibers need to induce cells to align as well as promote cell adhesion and encapsulation.
The researchers tested smooth and grooved GelMA fibers (see image) for cell alignment using myoblasts ― the building blocks of muscle engineering ― and found that the grooved fibers induced greater myoblast alignment than the smooth fibers. They then tested grooved GelMA and alginate fibers for cell adhesion and encapsulation using myoblasts and bone-synthesizing cells called osteoblasts. In both cases, the GelMA fibers exhibited superior cell adhesion, encapsulation and viability. “The grooved microfeatures on the fiber both improve the cell–material interaction and induce cell alignment by topographical constraint,” explains Ostrovidov.
The scientists also demonstrated that grooved GelMA fibers could be used to ‘co-culture’ two different cell types — they encapsulated endothelial cells that typically line blood vessels within the fibers and seeded myoblasts on the fiber surfaces.
“These results really open the door to fabricating a hierarchical biological tissue with several layers, different cell types and tissue organization,” remarks Ostrovidov. “We will pursue the development of hierarchical tissues in the future.”
Shi, X., Ostrovidov, S., Zhao, Y., Liang, X., Kasuya, M., Kurihara, K., Nakajima, K., Bae, H., Wu, H. & Khademhosseini, A. Microfluidic spinning of cell-responsive grooved microfibers. Advanced Functional Materials 25, 2250–2259 (2015). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.