

03/30/2015
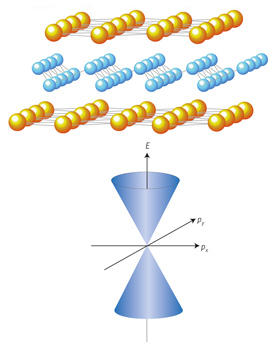
Top: Modified, with permission, from Ref. 1 © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Bottom: Reproduced, with permission, from Pesin, D. & MacDonald, A. H. “Spintronics and pseudospintronics in graphene and topological insulators.” Nature Materials 11, 409–416 (2012).
AIMR researchers have, for the first time, unambiguously shown that the electronic properties of silicene resemble those of its famous carbon cousin — graphene. In particular, they have demonstrated that the band structure of silicene contains a ‘massless Dirac cone’, making the material promising for use in high-speed electronic devices.
Silicene is a very attractive material on paper but has proven difficult to make in the lab. It is a single layer of silicon atoms arranged in a hexagonal honeycomb structure, similar to that of graphene. Unlike graphene, silicene is not completely flat; rather, it has a buckled structure — something that is both a blessing and a curse.
Silicene’s buckled structure is predicted to lead to attractive electronic properties, including a bandgap that can be tuned by applying a voltage perpendicular to the silicene sheet. Buckling, however, also makes silicene inherently unstable; consequently, no one has successfully produced a single layer of free-standing silicene.
Now, a team led by Takashi Takahashi of the AIMR at Tohoku University has produced the next best thing — a compound in which silicene layers are sandwiched between flat layers of calcium atoms (see top of image)1. They then investigated the electronic properties of the compound’s silicene layers using an analytical technique known as angle-resolved photoemission spectroscopy. When they did so, they obtained conclusive evidence that the silicene layers have a massless Dirac cone.
The electronic band structure of graphene consists of two circular cones whose tips touch at the origin (see bottom of image). These so-called Dirac cones endow graphene with special electronic properties that differ from those of simple insulators and conductors. In particular, when the Dirac cones are massless with no bandgap, electrons on the Dirac cone can move very rapidly in materials. Now, silicene has been shown to have the same band structure, confirming theoretical predictions of its electronic properties.
“Theoretical calculations had predicted that silicene has a massless Dirac cone, but it had not been experimentally confirmed whether there is a Dirac cone in silicene, and if it exists, if it is massless or massive,” explains Takahashi. “This is because early studies used ‘silicene’ samples fabricated on a metal substrate and thus could not eliminate the interaction with the substrate.”
The researchers hope to go a step further and “synthesize a genuine silicene sheet free from extrinsic components such as a substrate and measure the intrinsic electronic structure of silicene,” says Takahashi. They also plan to explore germanene, the germanium equivalent of graphene.
Noguchi, E., Sugawara, K., Yaokawa, R., Hitosugi, T., Nakano, H. & Takahashi, T. Direct observation of Dirac cone in multilayer silicene intercalation compound CaSi2. Advanced Materials 27, 856−860 (2015). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.