

02/23/2015
Modified, with permission, from Ref. 1 © 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
A metal-free catalyst based on graphene that can be used to produce low-cost, high-efficiency hydrogen fuel cells has been developed by AIMR researchers1. This will help realize the goal of a ‘hydrogen society’ ― one powered by hydrogen rather than fossil fuel or nuclear power.
Japan is enthusiastically embracing the concept of a hydrogen society, with the Japan Science and Technology Agency noting the importance of developing hydrogen-based fuel cells that could be used to power everything from vehicles to domestic residences and commercial activities. Yoshikazu Ito and Mingwei Chen from the AIMR at Tohoku University believe that “the clean energy of hydrogen will be a central energy target in the twenty-second century.”
Hydrogen fuel cells produce electricity via two electrochemical reactions ― the oxygen reduction reaction and the hydrogen evolution reaction ― both of which require a catalyst. The most effective catalysts tend to be noble metals such as platinum, but their superior performance comes with a prohibitive price tag. It is thus essential to develop low-cost, metal-free catalysts that have comparable catalytic activities to those of metals for both electrochemical reactions.
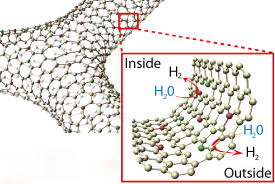
A promising contender is graphene ― a single layer of carbon atoms arranged in a honeycomb lattice. However, it is hindered by a relatively low chemical activity. In a previous study, Ito and colleagues succeeded in significantly boosting the rates of the oxygen reduction reaction by doping a three-dimensional interconnected network of graphene sheets with nitrogen2. Enhancing the hydrogen evolution reaction, however, has proven more challenging.
Inspired by their finding that sulfur was critical for catalyzing the hydrogen evolution reaction for a similar two-dimensional material, molybdenum disulphide3, Ito with other colleagues at AIMR and collaborators in China decided to try doping three-dimensional nanoporous graphene with both nitrogen and sulfur.
Nanoporous graphene generally contains various defects, including missing carbon atoms and dislocations in its lattice. Since defects tend to increase graphene’s chemical activity, the researchers used unmodified three-dimensional nanoporous graphene as a control to determine whether the increased catalytic activity could be solely explained by lattice defects. They discovered that the enhanced catalytic activity for the hydrogen evolution reaction resulted from the interplay between all three factors ― nitrogen, sulfur and defects.
The researchers are very excited about the potential of their catalyst. “Our metal-free hydrogen evolution reaction catalyst will contribute to the realization of a hydrogen society and hydrogen stations for fuel cell cars through enabling on-site hydrogen evolution,” predicts Ito.
Ito, Y., Cong, W., Fujita, T., Tang, Z. & Chen, M. W. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angewandte Chemie International Edition 53, 2131–2136 (2014). | article
Ito, Y., Qiu, H.-J., Fujita, T., Tanigaki, K. & Chen, M. Bicontinuous nanoporous N-doped graphene for the oxygen reduction reaction. Advanced Materials 26, 4145–4150 (2014). | article
Tan, Y. W., Liu, P., Chen, L. Y., Cong, W. T., Ito, Y., Han, J. H., Guo, X. W., Tang, Z., Fujita, T., Hirata, A. & Chen, M. W. Monolayer MoS2 films supported by 3D nanoporous metals for high-efficiency electrocatalytic hydrogen production. Advanced Materials 26, 8023–8028 (2014). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.