

09/29/2014
© 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
AIMR researchers have discovered how to unleash the promising catalytic activity of graphene by tweaking its chemical structure and twisting it into a three-dimensional (3D) nanoporous framework.
Fuel cells and lithium–air batteries generally use expensive components, such as platinum-based electrodes, to transform oxygen into electricity. Researchers have been considering graphene — a single layer of carbon atoms with metal-like properties — as a cheaper alternative. However, progress in this area had been hindered by graphene’s low chemical reactivity.
Now, Yoshikazu Ito, Mingwei Chen and colleagues from the AIMR at Tohoku University have developed an innovative way to turn flat graphene sheets into 3D objects using ‘bicontinuous’ nanoporous nickel templates1. These materials resemble a type of metallic sponge with flat surfaces infused with numerous nanoscale openings, known as nanopores. Chemical vapor deposition of benzene gas into the nickel nanopores, followed by acid treatment to remove the template, produced 3D graphene — a structure with an astonishingly high conductivity not far removed from that of its flat counterpart.
The team uncovered a connection between small pore size and high conductivity in 3D graphene and realized that the build-up of geometric defects needed to turn flat graphene into 3D nanopores was playing a role. Because these defect sites are electronically active, they conjectured that the sites could also serve as catalytic reaction sites.
But to engineer a specific catalytic response toward the ‘oxygen reduction reaction’ used to power fuel cells and lithium–air batteries, the researchers had to introduce foreign nitrogen dopant atoms into the graphene structure. To do so, they switched to pyridine gas — a benzene-like molecule with one nitrogen and five carbon atoms — and deposited it onto the bicontinuous nickel template.
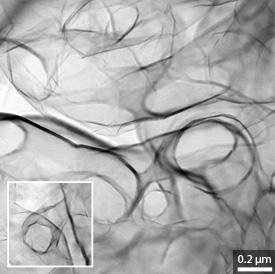
Analytical measurements revealed that the new, nitrogen-doped graphene also formed an ordered 3D framework with distinct nanopores (see image). When the researchers tested this material by plunging it into an oxygen-saturated electrolyte solution, they found it had superb catalytic behavior — particularly the samples with the tiniest possible pore sizes.
“Smaller pore structures require more geometric defects due to the high-curvature structures they impose,” explains Ito. “They also contain the highest pyridine nitrogen atom concentrations, and these dopant atoms create different kinds of geometric defects. This significantly enhances the nanoporous nitrogen-doped graphene’s catalytic activity.”
The team is now investigating how to further unleash the catalytic power of 3D graphene nanostructures by directing its activity toward the hydrogen evolution reaction that also plays a key role in batteries and fuel cells.
Ito, Y., Qiu, H.-J., Fujita, T., Tanabe, Y., Tanigaki, K. & Chen, M. Bicontinuous nanoporous N-doped graphene for the oxygen reduction reaction. Advanced Materials 26, 4145–4150 (2014). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.