

09/30/2013
Modified, with permission, from Ref. 1 © 2013 American Chemical Society
Mercury enters drinking water from a number of sources, including the erosion of natural deposits, wastewater discharged from oil refineries and runoff from landfill and agricultural land. As its accumulation in humans can cause irreversible damage to the brain and central nervous system, the levels in water are closely monitored.
“Although many methods have been reported, developing a quantitative approach capable of detecting sub-part-per-trillion levels of mercury ions with high selectivity is still challenging,” explains Ling Zhang from the AIMR at Tohoku University. Zhang, Mingwei Chen, Qi-Kun Xue and co-workers have now designed a nanoporous gold-based optical sensor that is around 1,000 times more sensitive than conventional optical methods1. “This is the most sensitive optical sensor for mercury ion detection in water known so far,” Xue says. “The US Environmental Protection Agency’s limit for mercury ions in drinking water is 10 nanomoles per liter, and the detection limit of our sensor is 10,000 times lower.”
The team’s sensor utilizes the technique of surface-enhanced resonance Raman scattering (SERRS). Like Raman spectroscopy, SERRS relies on the fact that every molecule scatters light differently — an effect that can be weak and difficult to detect. To overcome this issue, the Raman signal is amplified in two ways. First, a roughened metal surface — in this case nanoporous gold — is used. A laser excites the gold’s surface plasmons, which increases the surrounding electric field. As Raman scattering intensities are proportional to this electric field, the signal is therefore amplified. Second, the excitation wavelength of the laser is matched to the absorbance of the molecule being analyzed. Thus, the laser light is absorbed, further amplifying the signal.
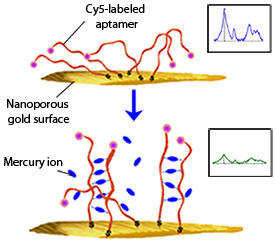
In the researchers’ study, the fluorochrome reporter molecule cyanine 5 (Cy5) is detected, rather than the mercury ion itself. Their sensor comprises flexible, single-stranded aptamers that are tagged with Cy5 and immobilized on the gold surface. Mercury ions, when present, bind to adjacent aptamers, pairing them to produce relatively rigid duplex-like structures (see image). This pulls the Cy5 tags up and away from the gold, resulting in a measurable reduction in the SERRS signal. At higher mercury concentrations, a greater number of aptamers are bound together, which further reduces the SERRS signal.
“Having successfully detected mercury ions in river water and underground water,” says Chen, “we are now working on the design and fabrication of a microchip that can be used in conjunction with a portable Raman spectrometer for real-world in situ detection.” The team is also looking to tweak the aptamers to test for other heavy metal ions.
Zhang, L., Chang, H., Hirata, A., Wu, H., Xue, Q.-K. & Chen, M. Nanoporous gold based optical sensor for sub-ppt detection of mercury ions. ACS Nano 7, 4595–4600 (2013). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.