

08/26/2013
© 2013 Akihiko Hirata
Most of the glasses that we encounter everyday are transparent and appear to be rather ordinary. A closer look, however, reveals an intriguing composition: glasses are basically frozen liquids, meaning that their atoms are randomly arranged. Researchers from the AIMR at Tohoku University and international collaborators have now shown that, surprisingly, the atoms of some glasses containing metallic components also display a local order that is based on icosahedral geometric structures1. “We provide the first direct experimental evidence of the existence of icosahedral order in metallic glasses,” explains Mingwei Chen, who led the research team to this discovery, which confirms previous theoretical predictions.
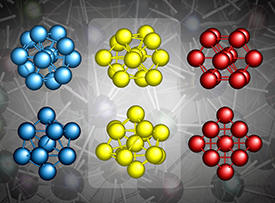
Atoms in metals behave like perfect spheres, forming perfect crystals with atomic arrangements in the form of face-centered cubic (fcc) or body-centered cubic (bcc) structures, for example. Some metallic compounds can also form glasses when cooled fast enough after melting. Scientists were unsure as to why some metals form glasses instead of crystallizing and assumed that upon fast cooling, atoms in the metal are prevented from forming a crystal when they arrange into icosahedra. Such arrangements are similar in appearance to fcc crystal structures but importantly cannot form large-scale, periodic structures (see image).
To study the structure of metallic glasses, Chen, Akihiko Hirata and colleagues used an angstrom-beam electron diffraction technique in which a tiny beam of electrons is guided onto a sample of glass that is only a few atoms wide. As the electrons pass through the glass, they are reflected by the atoms and subsequently hit a screen that records the pattern of their reflection. With the help of computer analysis, the positions of the atoms in the sample can be determined from these patterns.
While the researchers were able to confirm the icosahedral atomic structure of metallic glasses, they also found that the icosahedra were not perfectly formed, as previously assumed. Interestingly, this slight distortion occurs in a way that makes the icosahedra appear even closer in structure to the fcc arrangement.
Beyond the discovery of local order in metallic glasses, the Angstrom-beam electron diffraction technique itself is a powerful way of studying compounds on the atomic scale, comments Chen. “We have just reached the starting point to understand the true relationship between structure and properties of disordered materials. We now have a reliable experimental method to directly investigate local atomic structure, and in the present case to show the correlation between atomic-scale structure and glass formation, and perhaps even the structural origin of the glass transition.”
Hirata, A., Kang, L. J., Fujita, T., Klumov, B., Matsue, K., Kotani, M., Yavari, A. R. & Chen, M. W. Geometric frustration of icosahedron in metallic glasses. Science 341, 376–379 (2013). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.