

10/31/2011
Platinum-based materials are traditionally the catalysts of choice for incorporation in fuel cells. In recent years, however, palladium-based materials including bimetallic alloys have become more attractive due to their high electrocatalytic activity in key oxidation and reduction reactions. On the nanoscale, catalysts formed from palladium or platinum nanoparticles have shown high performance owing to their large surface areas and outstanding mechanical and electrical properties.
Mingwei Chen at the WPI Advanced Institute for Materials Research at Tohoku University, Japan, and colleagues have now prepared a bulk palladium-nickel alloy with a nanoporous structure that combines some of these advantages to produce an easily recyclable, low-cost and high-performance catalyst1. In a series of electrochemical studies, they have demonstrated that their novel nanoporous Pd–Ni alloy (np-PdNi) is a superior catalyst for small molecule electro-oxidation and oxygen reduction reactions.
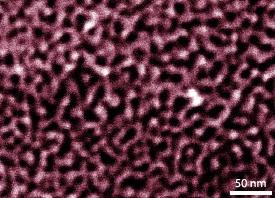
The np-PdNi material is made by electrochemically dealloying a Pd20Ni80 bimetallic alloy in a sulfuric acid solution. Nickel partially leaches out of the alloy through control of the etching potentials. The structure of np-PdNi comprises crystalline metallic ligaments and nanoporous channels in a bicontinuous arrangement (see image). A combination of X-ray photoelectron spectroscopy and ion etching techniques reveals that the distribution of nickel within the metallic ligaments varies with depth—the core is nickel-rich, whereas the shell is palladium-rich.
For the electro-oxidation reactions of methanol or formic acid, np-PdNi shows a higher catalytic activity than nanoporous pure palladium and commercial nanoparticulate Pd–C catalysts. In addition, the onset potential for catalytic activity is more negative for np-PdNi than for nanoporous Pd, which implies that the kinetics of the electro-oxidation reaction are enhanced. The np-PdNi material also exhibits excellent electrochemical stability—after 500 cycles of the electro-oxidation of methanol, the loss of electrocatalytic activity is only around 8%, a better result than that of commercial Pd–C catalysts.
“The current catalytic performance of the np-PdNi in the oxygen reduction reaction is just comparable to commercial platinum catalysts. In the future, we need to further optimize the morphology and composition of the np-PdNi to improve catalytic performance so that it surpasses the expensive platinum catalyst,” says Chen.
Potential applications of the material include the development of various green energy devices, including commercial fuel cells, hydrogen sensors and nanostructured electrodes. Chen and his coworkers are currently investigating how the control of dealloying potentials and alteration of the alloy precursors could be further extended to make an even wider range of nanoporous materials with compositions that can be finely tuned.
Chen, L., Guo, H., Fujita, T., Hirata, A, Zhang, W., Inoue, A. & Chen, M. Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electro-oxidation and oxygen reduction reactions. Advanced Functional Materials 22, 4364-4370 (2011) | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.