

01/31/2011
© 2010 Wiley-VCH
Gold prices have skyrocketed in the past few years, but a study by researchers from the WPI-AIMR and others at Tohoku University1 promises to make this precious metal even more valuable. Naoki Asao, Yoshinori Yamamoto and their co-workers have found that nanoporous gold — a material produced by selectively leaching silver atoms from a gold–silver alloy film — can catalyze an important step in the production of silicon-based polymers under milder and ‘greener’ conditions than those required in conventional methods.
In its bulk state, gold has a familiar luster that doesn’t tarnish or discolor because it is chemically un-reactive. At the nanoscale, however, the confined electrons of gold atoms can begin to oscillate together, imparting new colors to the metal. “When we make nanoporous gold with pores around 100 nanometers in size, it has a reddish gold color,” says Asao. “As the pore size becomes smaller, the color becomes deeper, clearly indicating a change in electronic properties from the original thin gold film.”
This altered electronic behavior of nanoscale gold also makes it an active catalyst: gold nanoparticles supported on a solid substrate are known to catalyze a variety of reactions, providing an alternative to many toxic reagents in industrial processes. Unfortunately, these materials suffer from a limited lifespan: eventually, gold nanoparticles agglomerate on the surface of the solid support and lose their reactivity. In light of this issue, attention has recently turned to nanoporous gold films.
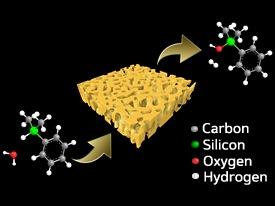
The researchers investigated the catalytic performance of nanoporous gold for the oxidation of organosilanes, which contain silicon–hydrogen bonds. On oxidation, a hydrogen atom on the silicon center is replaced by a hydroxyl group, making the molecule easier to combine into silicon-based polymers. However, the reactions typically used to achieve this result do not oxidize certain substrate types, tend to produce unwanted byproducts, or involve lengthy post-treatment processing.
The researchers found that immersing the nanoporous gold film in an aqueous organosilane solution at room temperature catalyzed the oxidation of silicon–hydrogen bonds with nearly quantitative efficiency (Fig. 1). Furthermore, the catalyst was easily recovered and reused several times with no change in either reactivity or its nanopore structure.
The researchers are now investigating the mechanisms of nanoporous gold catalysis, and expect that this approach will have a major impact in the field of sustainable chemistry. “We believe that nanoporous gold will be useful for molecular transformations of natural gas and bioethanol for green chemistry,” says Asao. “Nanoporous materials chemistry will surely develop much more in the future.”
Asao, N., Ishikawa, Y., Hatakeyama, N., Menggenbateer, Yamamoto, Y., Chen, M., Zhang, W. & Inoue, A. Nanostructured materials as catalysts: Nanoporous-gold-catalyzed oxidation of organosilanes with water. Angewandte Chemie International Edition 49, 10093–10095 (2010). | article
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.