

01/29/2018
© 2018 Katsuaki Sugawara
High-resolution spectroscopy by a team at the AIMR and Tohoku University has revealed how exposing graphene sheets — layers of carbon just one atom thick — to hydrogen gas under controlled conditions can transform them into fuel-storage devices as well as produce magnetically active energy states1.
Chemists have long known that inserting atoms and ions into layered crystals, a process known as intercalation, can enhance ordinary materials. For example, many rechargeable batteries rely on intercalation of lithium within graphite to achieve stable power generation. Because the effects of introduced atoms are expected to be heightened for single-layer graphene, researchers are actively working to create devices based on intercalation.
A natural target for graphene intercalation is hydrogen. That is because theory predicts it can create band gaps in high-conductivity graphene and produce the semiconductor-like properties needed for transistors and sensors. However, inserting lightweight hydrogen between carbon sheets requires careful adjustment, notes Katsuaki Sugawara from the AIMR.
“Because hydrogen’s radius and bond lengths are so much smaller than those of graphene, it’s hard to make it stay between the graphene layers,” he says. “We worked to fix that by tuning the conditions of hydrogen exposure.”
Sugawara and his team explored this problem using an analytical technique known as angle-resolved photoemission spectroscopy, which probes the electronic energy bands that hold solids together and shape their conductivity. By growing single graphene layers on silicon carbide wafers and pumping in hydrogen gas for specific times, they aimed to monitor changes to band gaps induced by the hydrogen atoms.
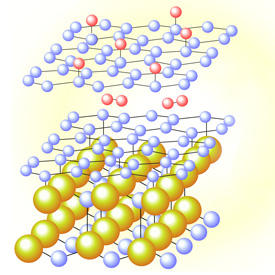
After optimizing temperatures for intercalation, the researchers observed that in pristine samples a new buffer layer of meshed carbon atoms appeared between the outer graphene sheet and the silicon carbide wafer. But longer exposures to hydrogen reduced the intensity of the buffer-layer energy bands and made bands associated with aromatic bonding in graphene more prominent — clear evidence that hydrogen atoms had entered between the layers and were disrupting existing chemical links (see image).
Further analysis of the band structure revealed that hydrogen exposure not only increased the available band gap for ballistic devices, but also created electronic states within the gap region. These states could tune the efficiency of ultrathin hydrogen fuel-storage devices or form a foundation for spintronic transistors, notes Sugawara.
“Theory predicts that the new gap states can introduce magnetic properties to hydrogen-adsorbed graphene,” he states. “We want to elucidate which hydrogen additions are ferromagnetic and which lead to insulators.”
This research highlight has been approved by the authors of the original article and all information and data contained within has been provided by said authors.